Current Management of Gastrointestinal Stromal Tumor GIST Joint










- Slides: 29

Current Management of Gastrointestinal Stromal Tumor (GIST) Joint Hospital Surgical Grand Round Dr. Tony Cheung PYNEH

Gastrointestinal Stromal Tumor n n n Neoplasm of interstitial Cajal cells 3000 -6000 cases/ year in the US Equal prevalence in male and female


Landmark discovery n 1998 q Majority of GIST have oncogenic gain-of-function mutations of the KIT receptor tyrosine kinase Hirota S et al. Science 1998; 279: 577– 580. n 2001 q Imatinib (Gleevec) n KIT tyrosine kinase inhibitor (TKI) Joensuu et al. N Engl J Med 2001; 1052: 1052– 1056.

OGD CXR EUS CT PET

Investigations n n n CXR OGD EUS q look for size, irregular borders, echogenic foci, cystic spaces Gastrointest Endosc 2003; 57: 469– 474. Med Clin North Am 2005; 89: 139– 158, viii. n Contrast CT q q n for size and anatomical location determine features of GIST – well vascularized, necrotic centre, heterogeneous appearance PET q q identify metastatic disease monitor response to medical tx

Risk Stratification of GIST Miettinen et al. Am J Surg Pathol 2005; 29: 52– 68. Classification Size Mitotic rate (/50 HPF) Prognosis Benign ≤ 2 cm ≤ 5 No tumor related mortality Very low malignant potential 2 -10 cm <5 < 3% recurrence Uncertain or low malignant potential ≤ 2 cm >5 No reported recurrence Low to moderate malignant potential > 10 cm or 2 -5 cm ≤ 5 or >5 12 -15% >5 49 -86% High malignant potential > 5 cm

Management n Primary GIST n Advanced / Metastatic GIST

Localized GIST n Goal of operation q q n n Complete macroscopic resection with an intact pseudocapsule Negative microscopic margin (R 0 resection) If tumor rupture associated with high risk of intraabdominal dissemination of tumor cells and recurrence However, no additional benefit of wide resection of gastric GIST to obtain generous negative resection margin

Laparotomy OR Laparoscopy

Laparotomy n R 0 resection options q q q n Wedge resection, segmental resection Extensive resection En bloc contiguous visceral resection Method of choice for all non-gastric GISTs

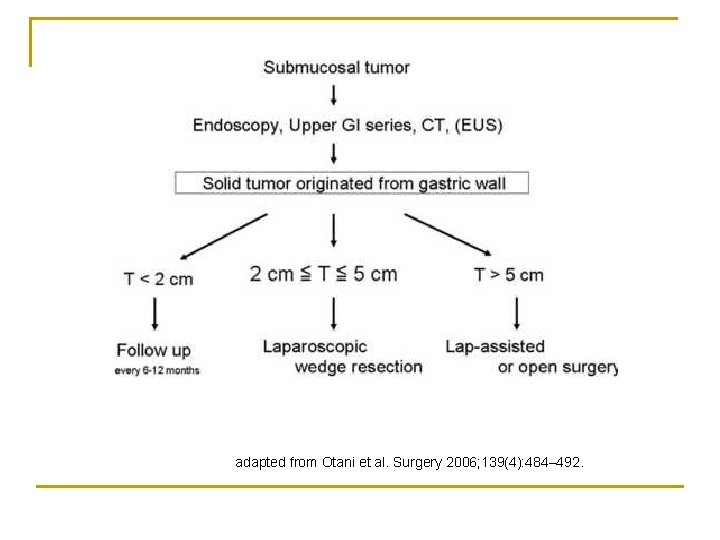
Laparoscopy n n n < 5 cm tumor for stomach GIST Laparoscopic wedge resections of stomach GIST Otani et al. Surgery 2006; 139(4): 484– 492. No series on long term outcome with laparoscopy for non-stomach GIST

adapted from Otani et al. Surgery 2006; 139(4): 484– 492.

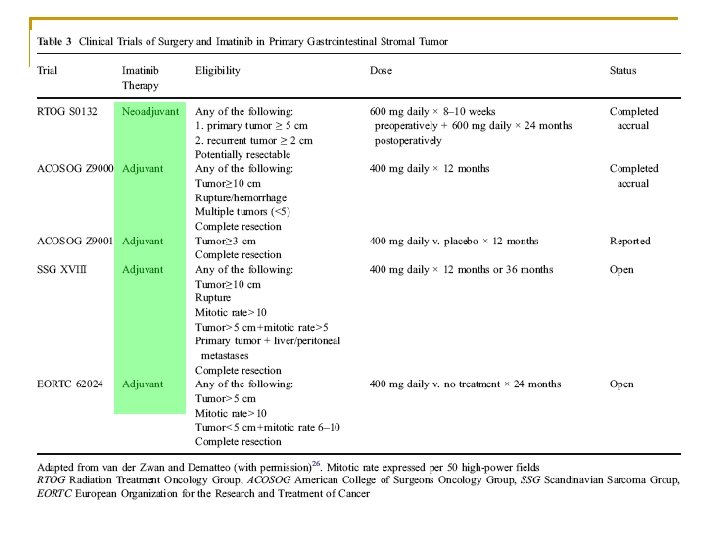
But… n 5 -year overall survival despite negative resection margin 42 -52% Crosby et al. Ann Surg Oncol 2001; 8(1): 50– 59. Neoadjuvant or adjuvant use of tyrosine kinase inhibitors


Advanced / Metastatic GIST 1. 2. Imatinib alone Imatinib + Cytoreductive surgery

Nature of GIST n Metastatic spread q q q n peritoneal cavity liver uncommonly regional lymph nodes Large GISTs tend to displace rather than invade adjacent organs Miettinen et al. Am J Surg Pathol 2005; 29: 52– 68.

Imatinib alone Outcome n 1. 2. 3. Response Primary resistance Secondary resistance

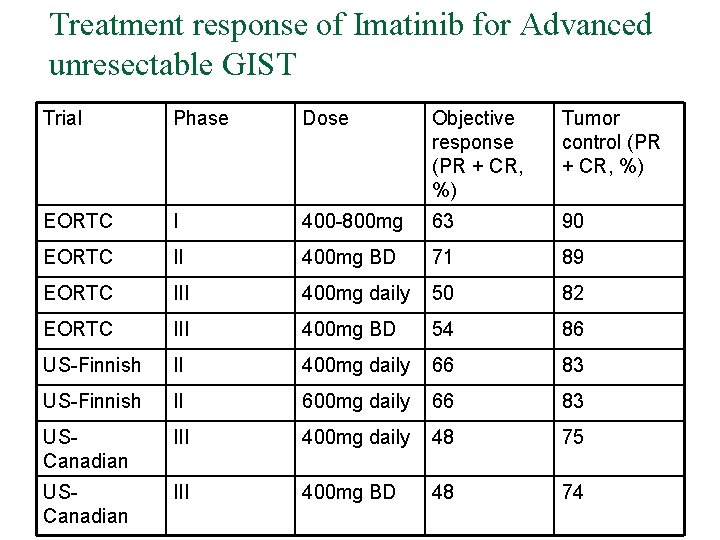
Treatment response of Imatinib for Advanced unresectable GIST Trial Phase Dose Objective response (PR + CR, %) Tumor control (PR + CR, %) EORTC I 400 -800 mg 63 90 EORTC II 400 mg BD 71 89 EORTC III 400 mg daily 50 82 EORTC III 400 mg BD 54 86 US-Finnish II 400 mg daily 66 83 US-Finnish II 600 mg daily 66 83 USCanadian III 400 mg daily 48 75 USCanadian III 400 mg BD 48 74

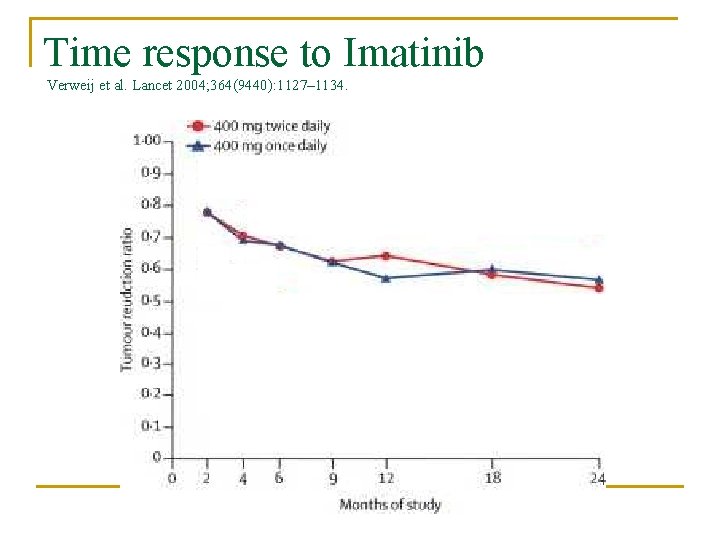
Time response to Imatinib Verweij et al. Lancet 2004; 364(9440): 1127– 1134.

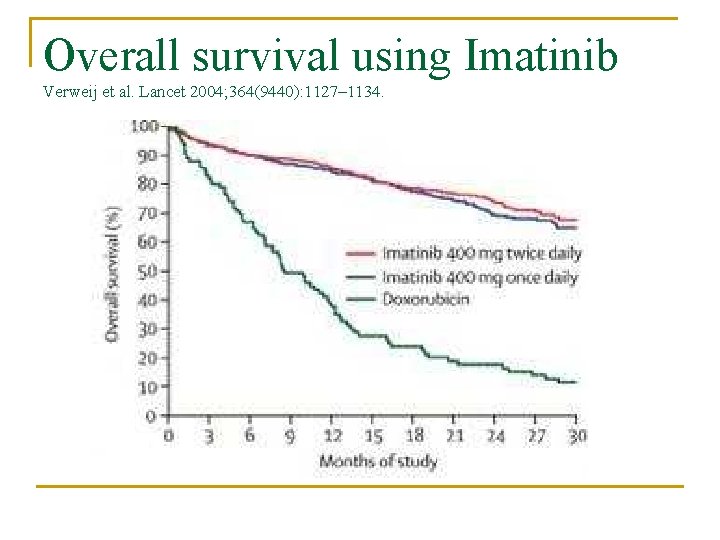
Overall survival using Imatinib Verweij et al. Lancet 2004; 364(9440): 1127– 1134.

Response Overall disease control in 70 -85% of patient n Median progression-free survival is 2024 months n Overall survival time following imatinib therapy > 36 months n

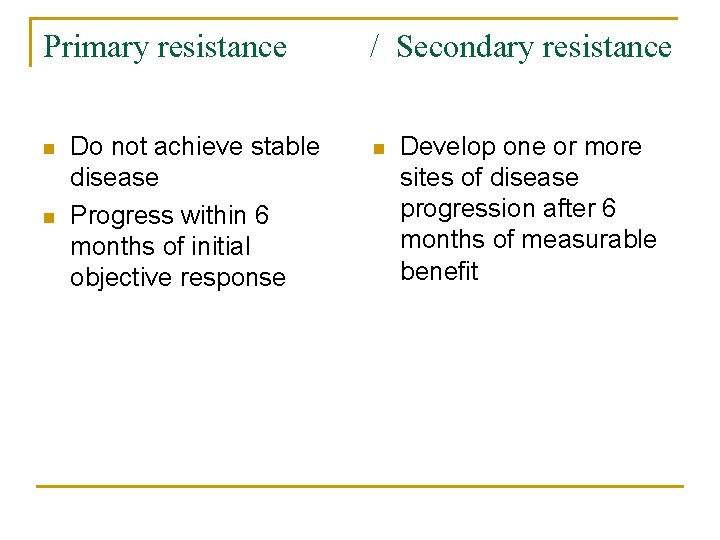
Primary resistance n n Do not achieve stable disease Progress within 6 months of initial objective response / Secondary resistance n Develop one or more sites of disease progression after 6 months of measurable benefit

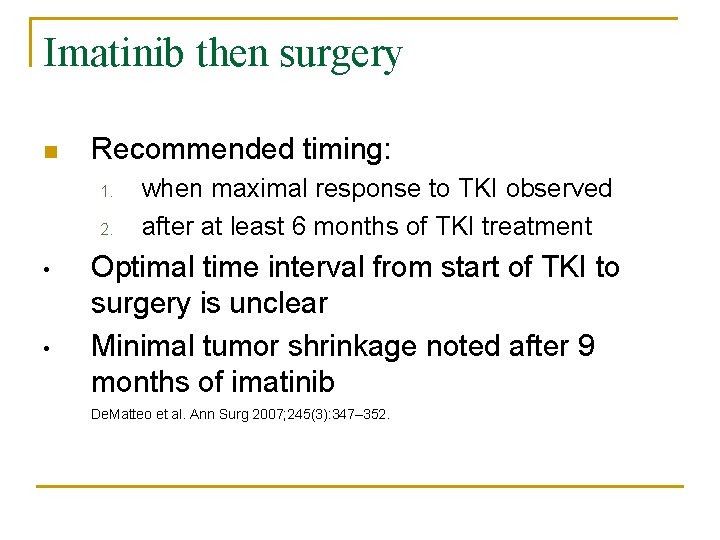
Imatinib then surgery n Recommended timing: 1. 2. • • when maximal response to TKI observed after at least 6 months of TKI treatment Optimal time interval from start of TKI to surgery is unclear Minimal tumor shrinkage noted after 9 months of imatinib De. Matteo et al. Ann Surg 2007; 245(3): 347– 352.

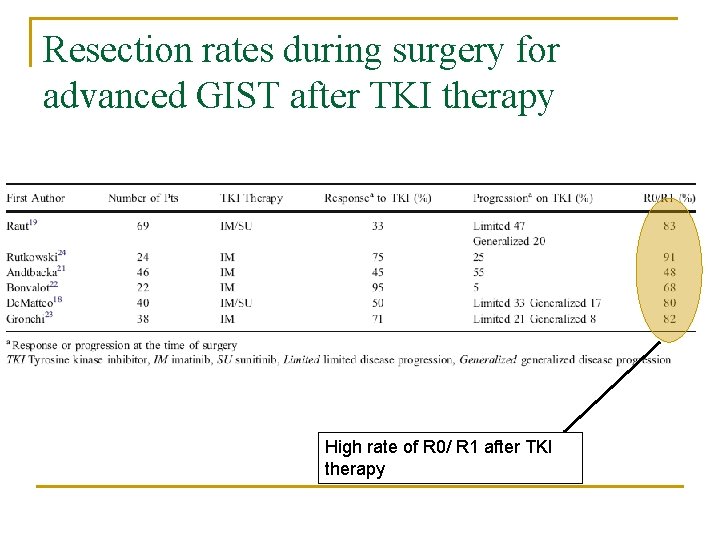
Resection rates during surgery for advanced GIST after TKI therapy High rate of R 0/ R 1 after TKI therapy

• Surgical candidates • • ongoing response limited disease progression evolving necrosis or impending emergency Non surgical candidates • generalized progression

Surveillance n n Surveillance CT thorax/ abdomen/ pelvis q 36 months for 1 st 5 years, then annually PET not routinely needed Chandrajit et al. J Gastrointest Surg (2008) 12: 1592– 1599

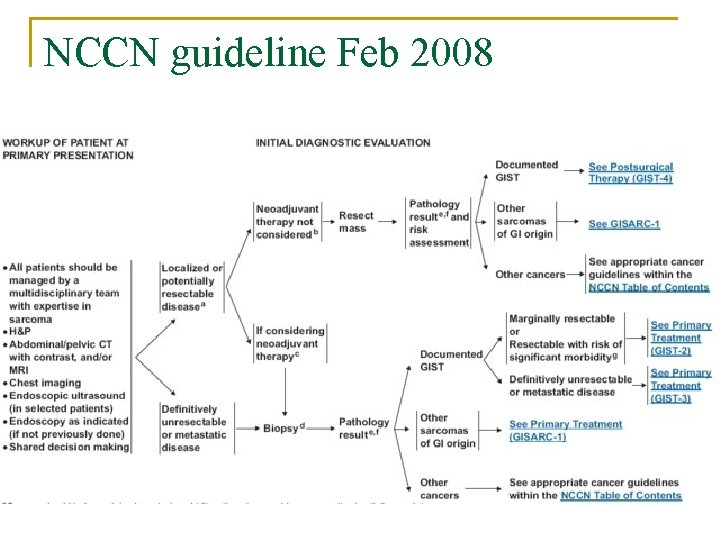
NCCN guideline Feb 2008

Thank you!
 Seks kord stromal tümör
Seks kord stromal tümör Stivarga side effects
Stivarga side effects Hikmet hassa
Hikmet hassa Borborygmi
Borborygmi The ride stuff readworks answer key
The ride stuff readworks answer key Exegetic translation
Exegetic translation Maus book 1 summary
Maus book 1 summary What is a 20 word gist
What is a 20 word gist When was sequoyah born and died
When was sequoyah born and died Chapters of to kill a mockingbird
Chapters of to kill a mockingbird Gist
Gist Gist review
Gist review What is the gist of pages 6 12 in unbroken
What is the gist of pages 6 12 in unbroken Embriologia del sistema gastrointestinal
Embriologia del sistema gastrointestinal Gastrointestinal tract
Gastrointestinal tract Chemotrypsinogen
Chemotrypsinogen Emt chapter 18 gastrointestinal and urologic emergencies
Emt chapter 18 gastrointestinal and urologic emergencies Chapter 15 the gastrointestinal system
Chapter 15 the gastrointestinal system Gastrointestinal hormones
Gastrointestinal hormones Dr sigid djuniawan
Dr sigid djuniawan Sinal de lichtenberg medicina legal
Sinal de lichtenberg medicina legal Verner morrison sendromu
Verner morrison sendromu Gastrointestinal medical terminology breakdown
Gastrointestinal medical terminology breakdown Nutrition focused physical assessment
Nutrition focused physical assessment Foregut midgut hindgut
Foregut midgut hindgut Gastrointestinal tract
Gastrointestinal tract Motilidad gastrointestinal
Motilidad gastrointestinal Upper gi bleeding management
Upper gi bleeding management Arteria e veia
Arteria e veia Corte sagital mediano
Corte sagital mediano