current cathodic ic potential potential V V current









- Slides: 49

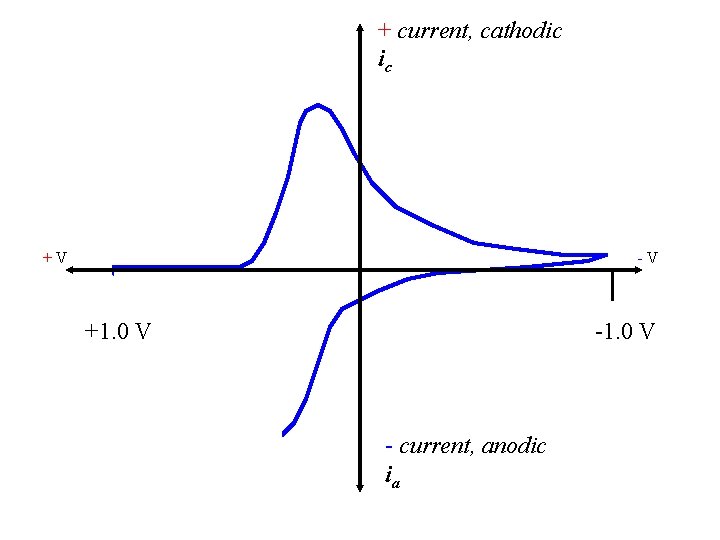
+ current, cathodic ic + potential, - potential, V V - current, anodic ia

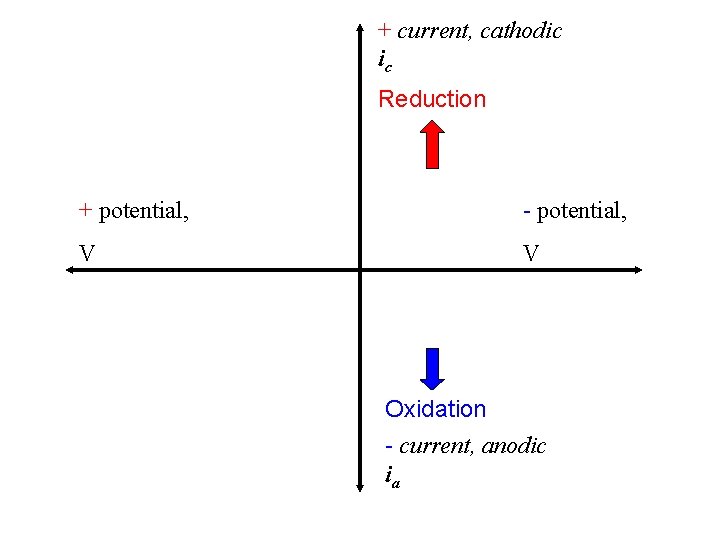
+ current, cathodic ic Reduction + potential, - potential, V V Oxidation - current, anodic ia

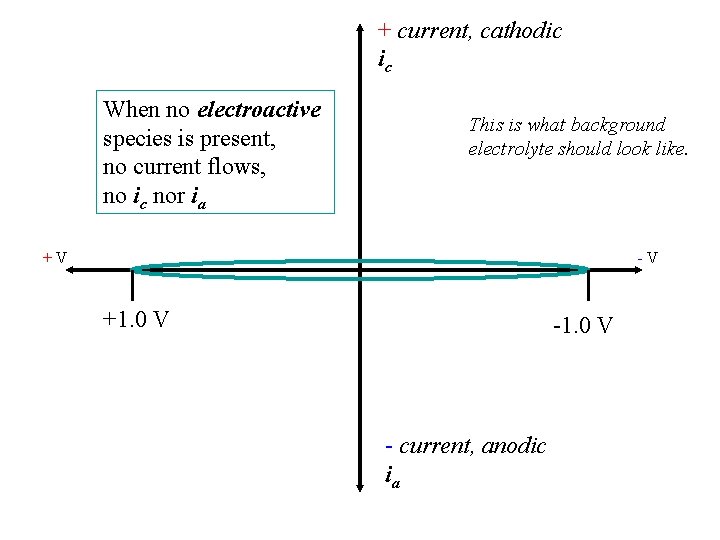
+ current, cathodic ic When no electroactive species is present, no current flows, no ic nor ia This is what background electrolyte should look like. +V -V +1. 0 V - current, anodic ia

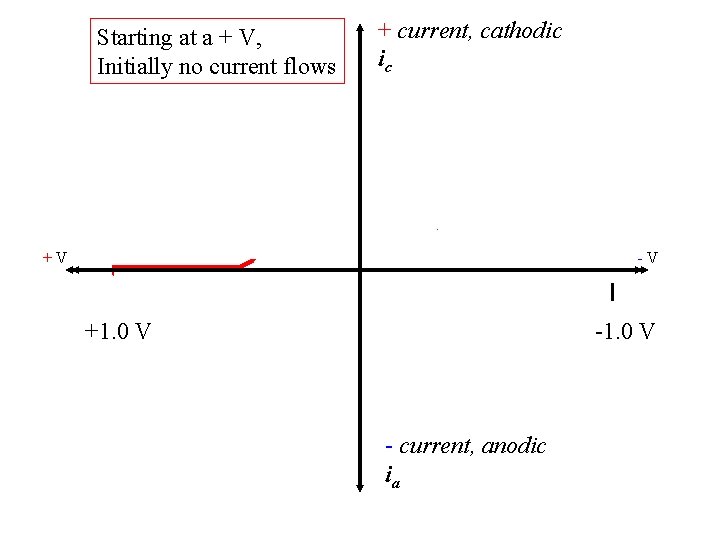
Starting at a + V, Initially no current flows + current, cathodic ic +V -V +1. 0 V - current, anodic ia

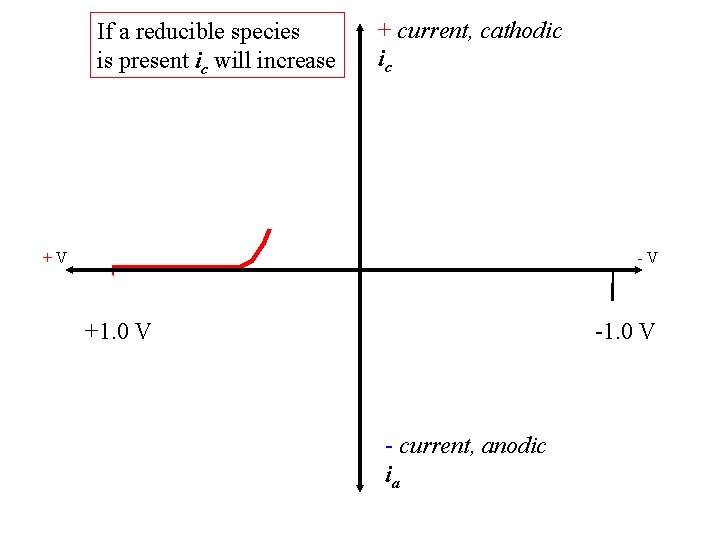
If a reducible species is present ic will increase + current, cathodic ic +V -V +1. 0 V - current, anodic ia

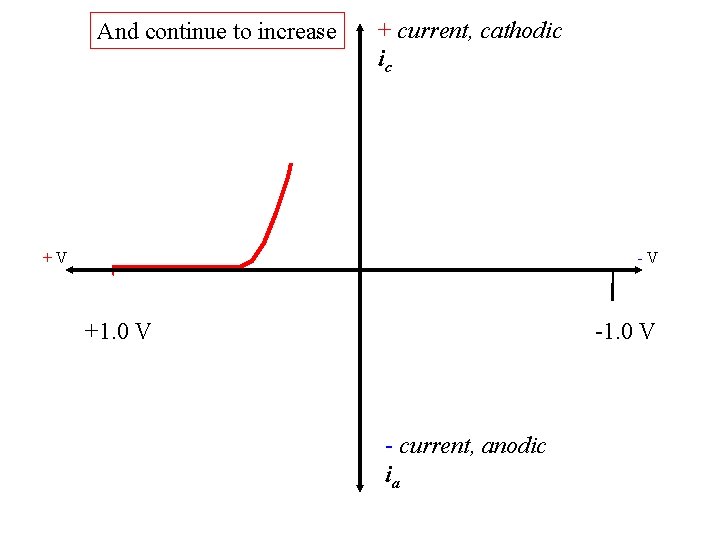
And continue to increase + current, cathodic ic +V -V +1. 0 V - current, anodic ia

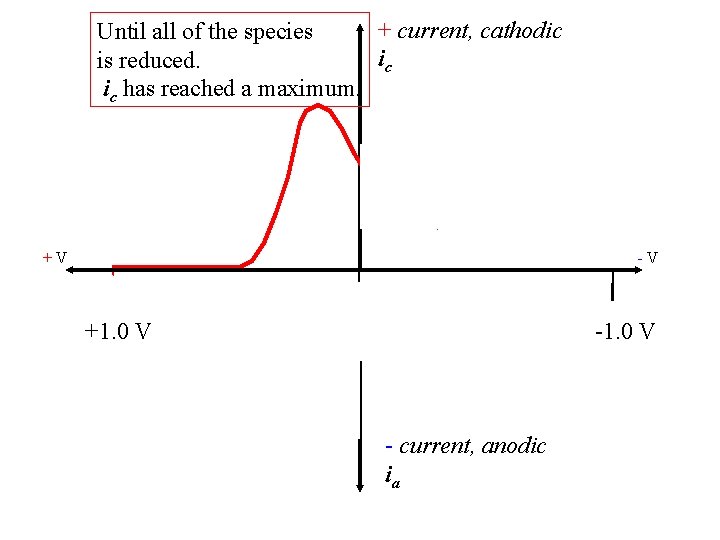
+ current, cathodic Until all of the species ic is reduced. ic has reached a maximum. +V -V +1. 0 V - current, anodic ia

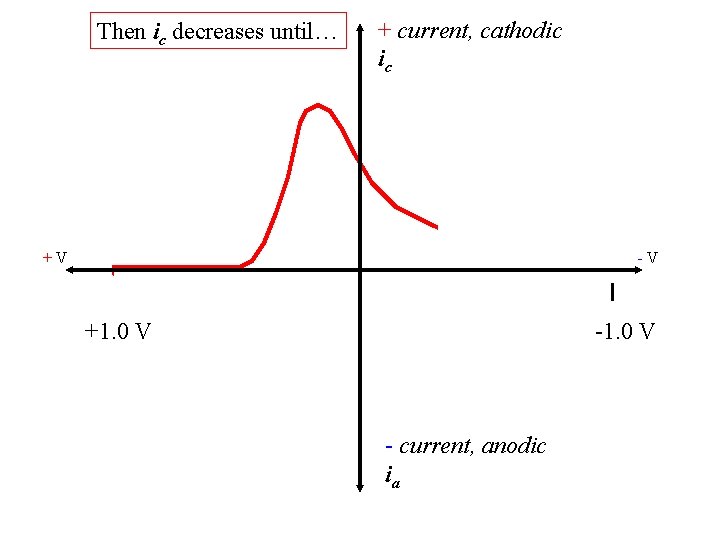
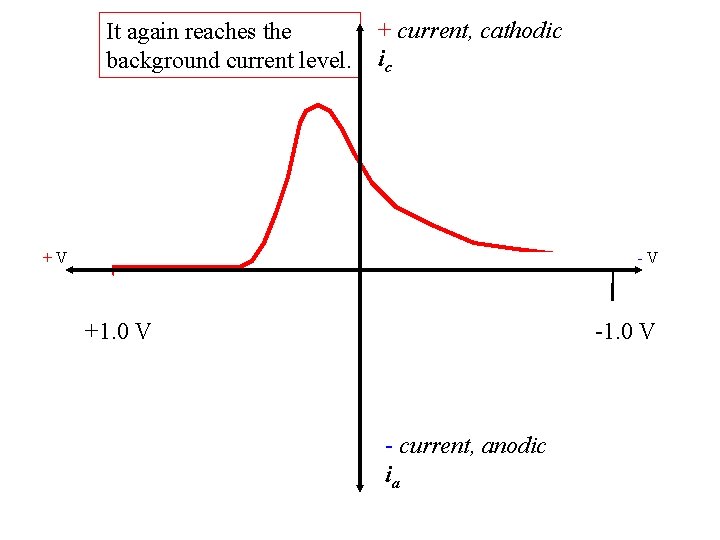
Then ic decreases until… + current, cathodic ic +V -V +1. 0 V - current, anodic ia

It again reaches the background current level. + current, cathodic ic +V -V +1. 0 V - current, anodic ia

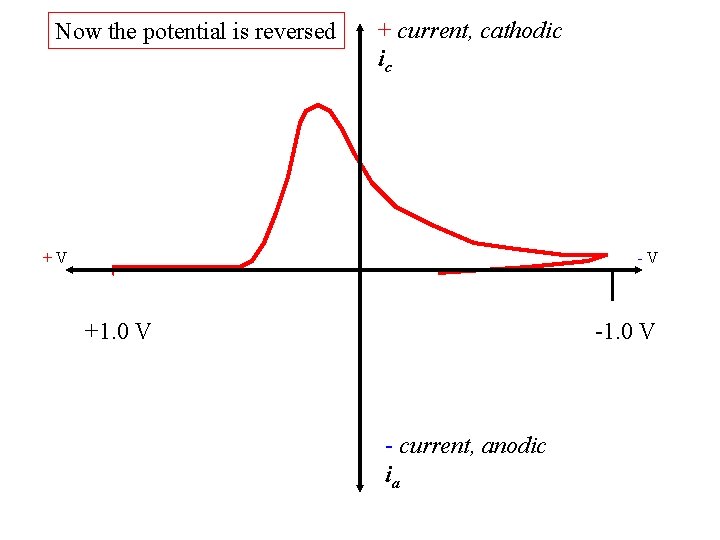
Now the potential is reversed + current, cathodic ic +V -V +1. 0 V - current, anodic ia

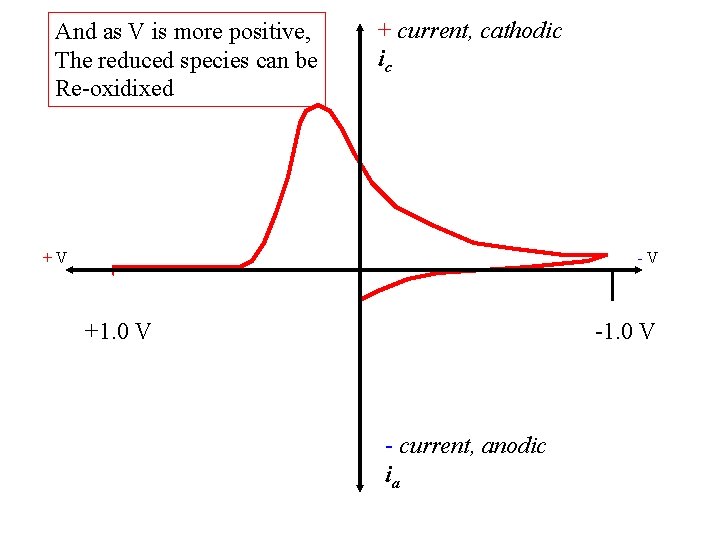
And as V is more positive, The reduced species can be Re-oxidixed + current, cathodic ic +V -V +1. 0 V - current, anodic ia

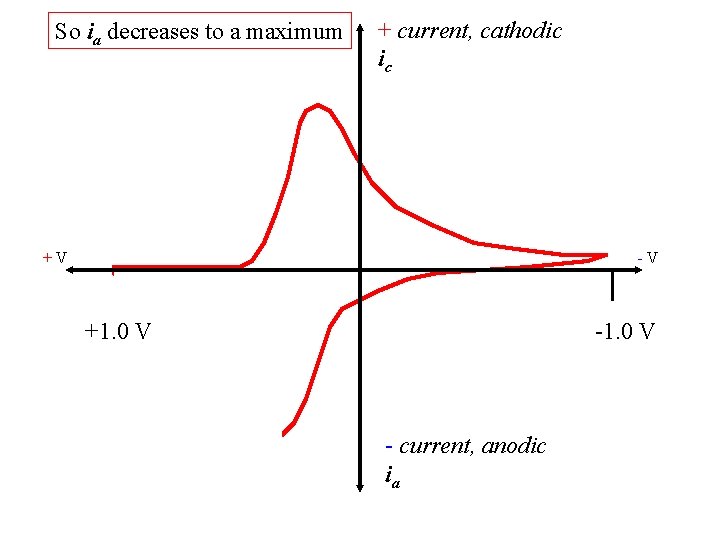
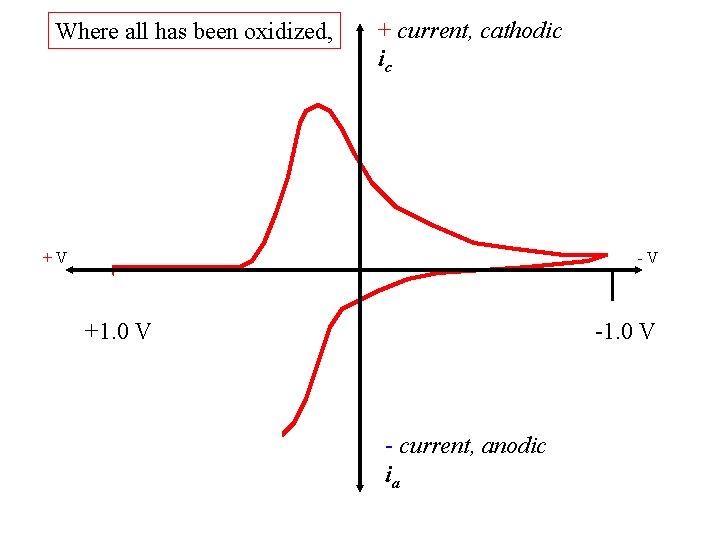
So ia decreases to a maximum + current, cathodic ic +V -V +1. 0 V - current, anodic ia

Where all has been oxidized, + current, cathodic ic +V -V +1. 0 V - current, anodic ia

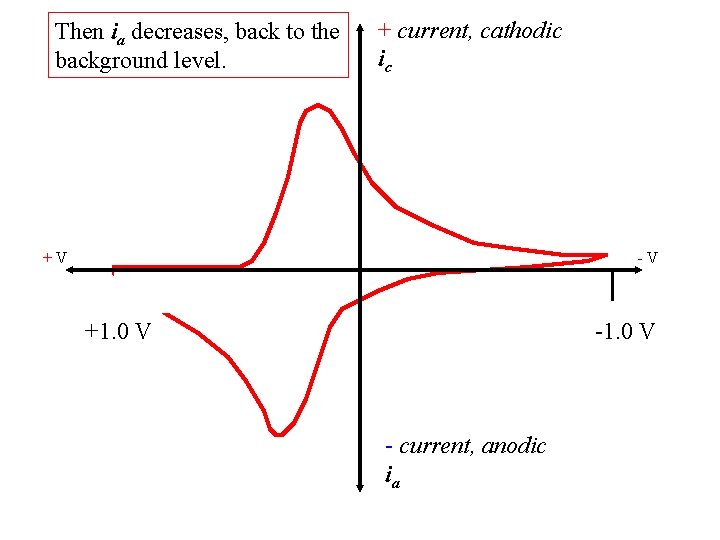
Then ia decreases, back to the background level. + current, cathodic ic +V -V +1. 0 V - current, anodic ia

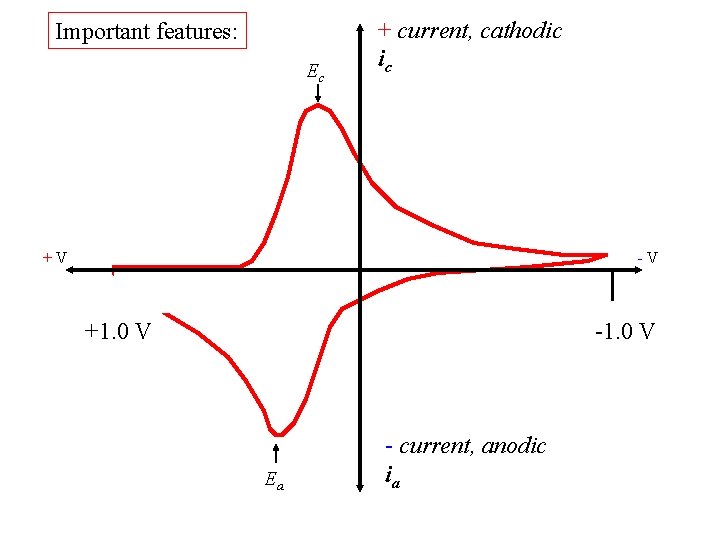
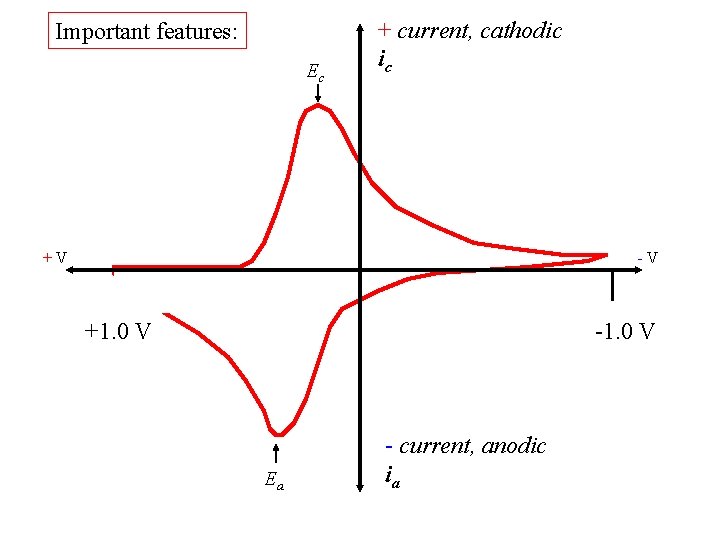
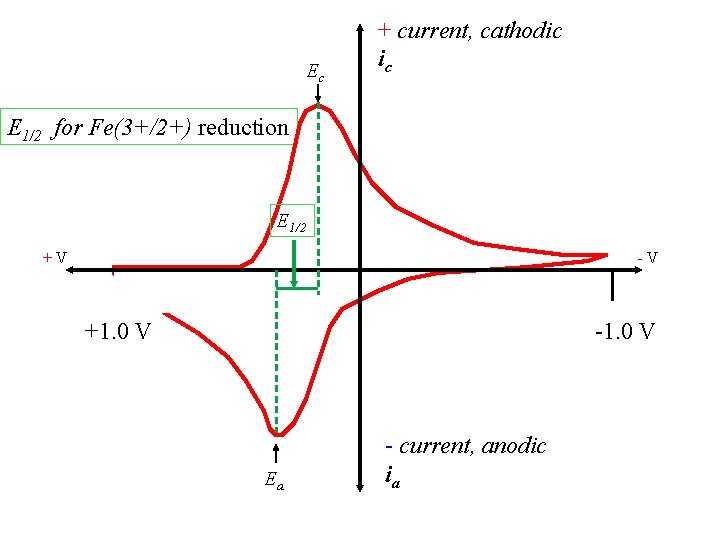
Important features: Ec + current, cathodic ic +V -V +1. 0 V -1. 0 V Ea - current, anodic ia

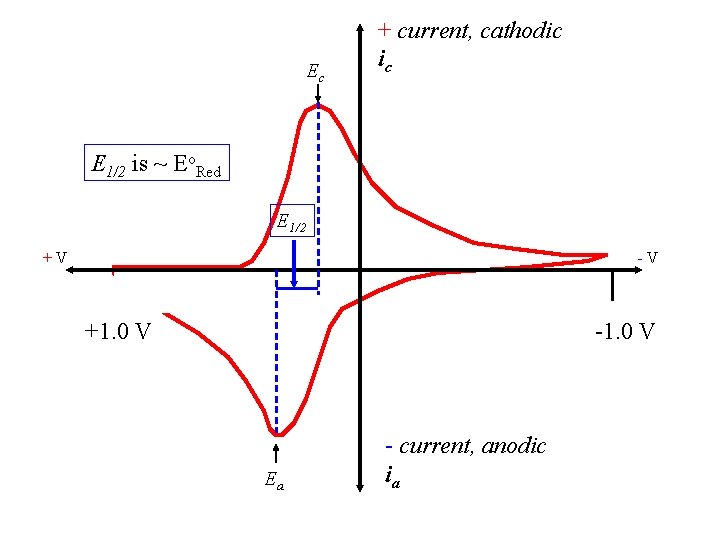
Ec + current, cathodic ic E 1/2 is ~ Eo. Red E 1/2 +V -V +1. 0 V -1. 0 V Ea - current, anodic ia

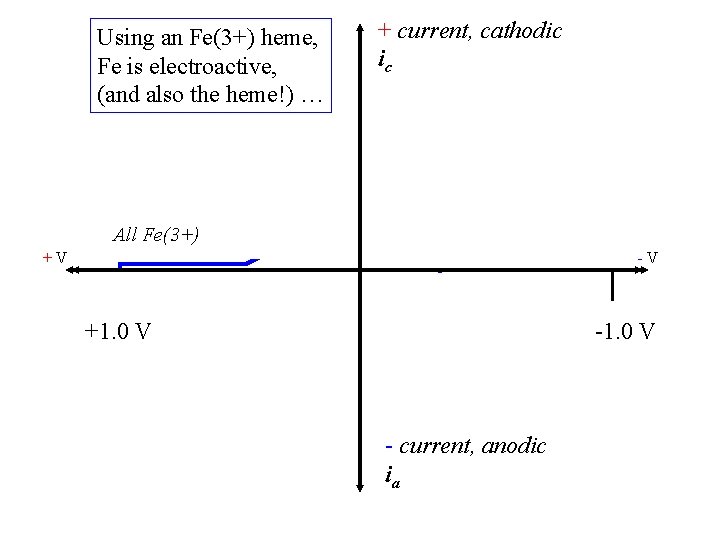
Using an Fe(3+) heme, Fe is electroactive, (and also the heme!) … + current, cathodic ic All Fe(3+) +V -V +1. 0 V - current, anodic ia

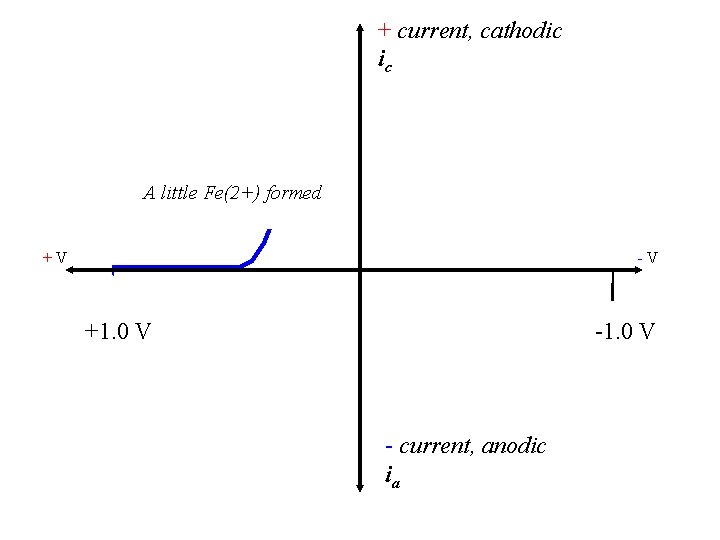
+ current, cathodic ic A little Fe(2+) formed +V -V +1. 0 V - current, anodic ia

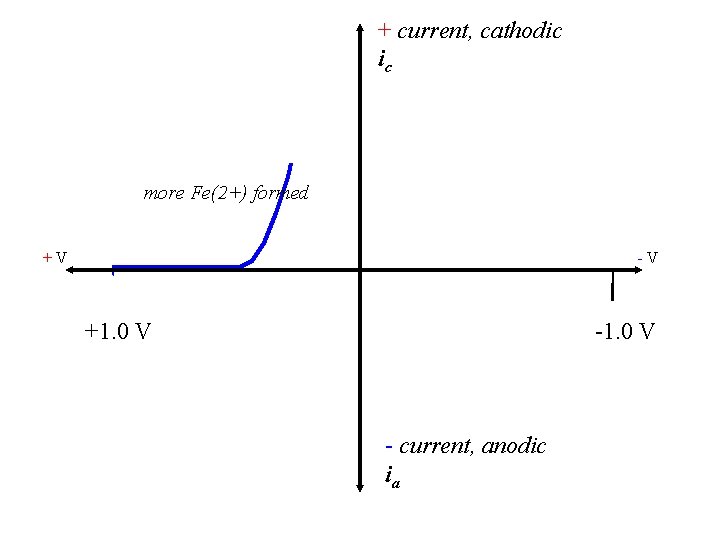
+ current, cathodic ic more Fe(2+) formed +V -V +1. 0 V - current, anodic ia

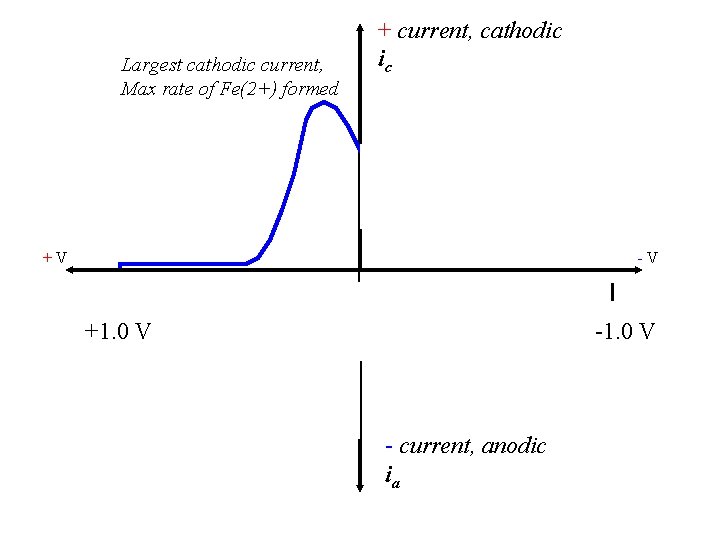
Largest cathodic current, Max rate of Fe(2+) formed + current, cathodic ic +V -V +1. 0 V - current, anodic ia

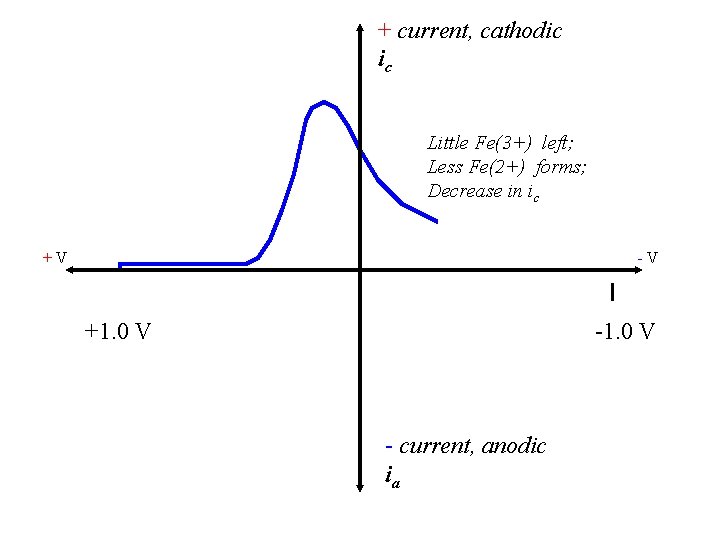
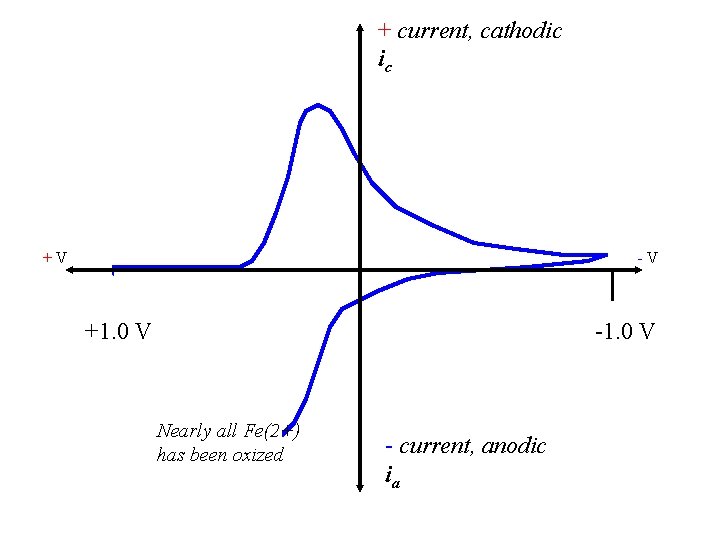
+ current, cathodic ic Little Fe(3+) left; Less Fe(2+) forms; Decrease in ic +V -V +1. 0 V - current, anodic ia

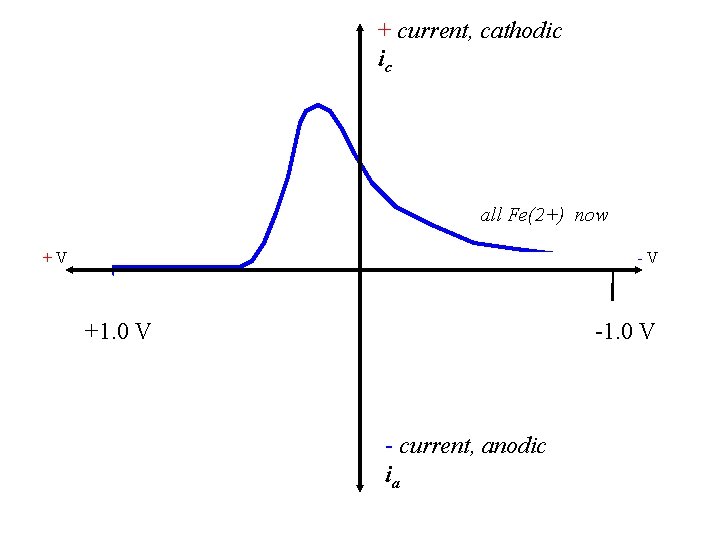
+ current, cathodic ic all Fe(2+) now +V -V +1. 0 V - current, anodic ia

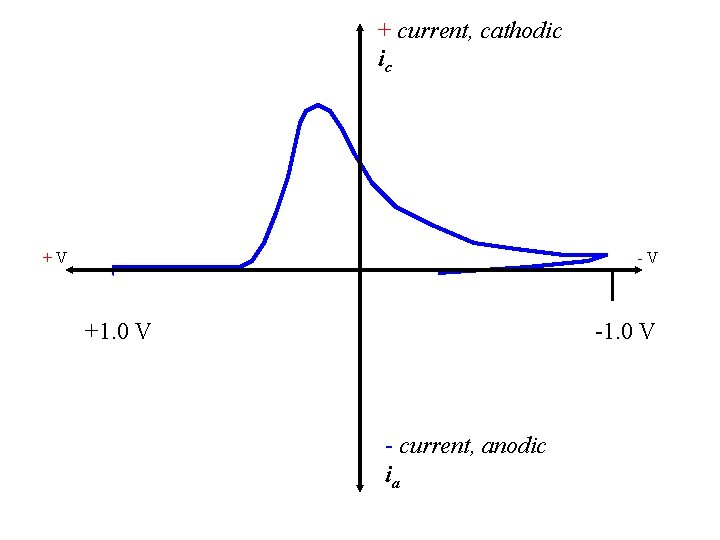
+ current, cathodic ic +V -V +1. 0 V - current, anodic ia

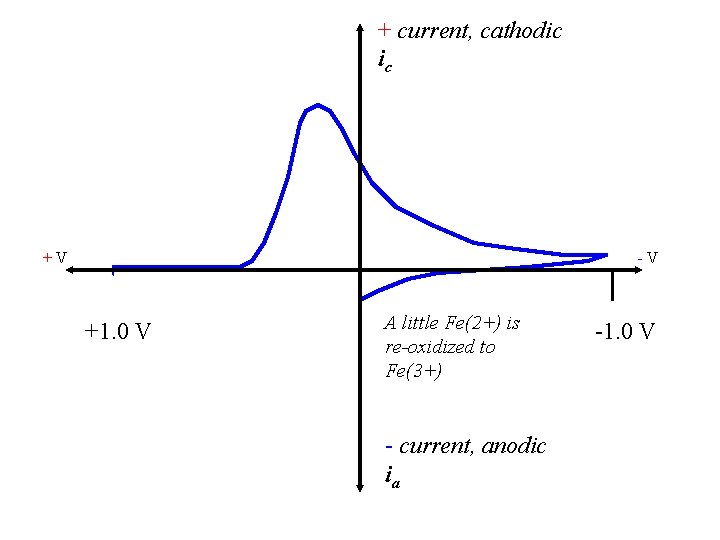
+ current, cathodic ic +V -V +1. 0 V A little Fe(2+) is re-oxidized to Fe(3+) - current, anodic ia -1. 0 V

+ current, cathodic ic +V -V +1. 0 V - current, anodic ia

+ current, cathodic ic +V -V +1. 0 V -1. 0 V Nearly all Fe(2+) has been oxized - current, anodic ia

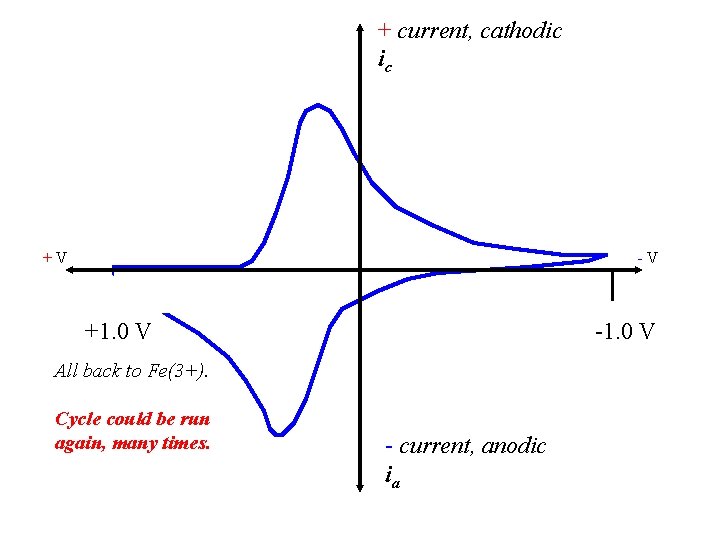
+ current, cathodic ic +V -V +1. 0 V -1. 0 V All back to Fe(3+). Cycle could be run again, many times. - current, anodic ia

Important features: Ec + current, cathodic ic +V -V +1. 0 V -1. 0 V Ea - current, anodic ia

Ec + current, cathodic ic E 1/2 for Fe(3+/2+) reduction E 1/2 +V -V +1. 0 V -1. 0 V Ea - current, anodic ia

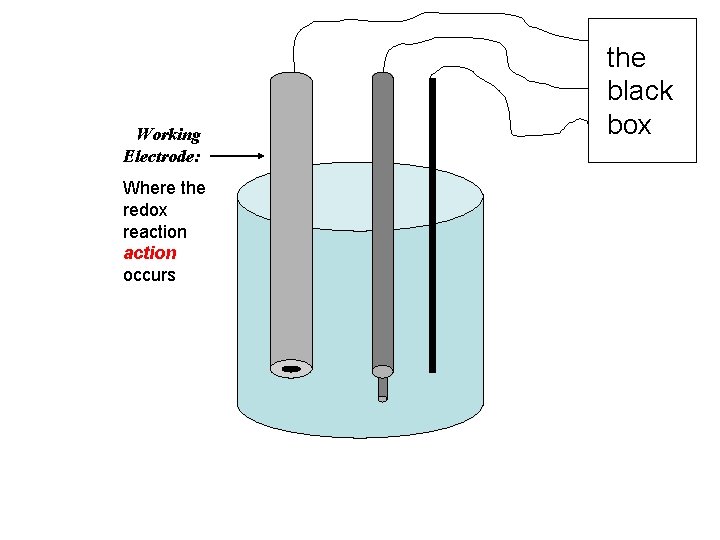
Working Electrode: Where the redox reaction occurs the black box

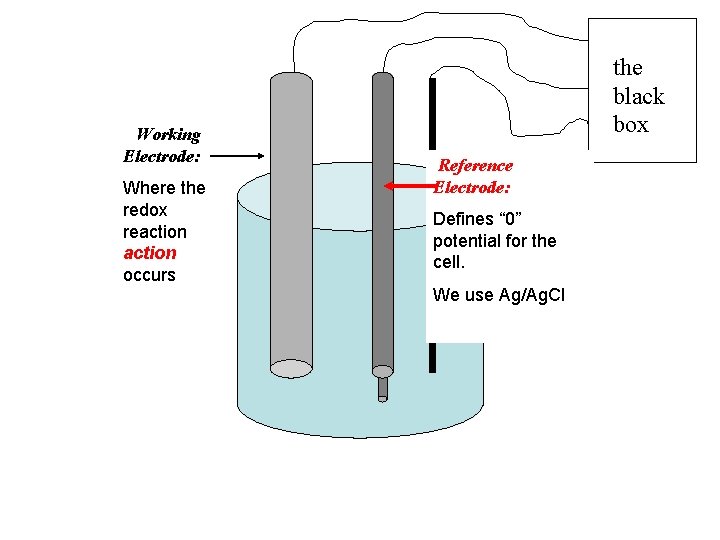
Working Electrode: Where the redox reaction occurs the black box Reference Electrode: Defines “ 0” potential for the cell. We use Ag/Ag. Cl

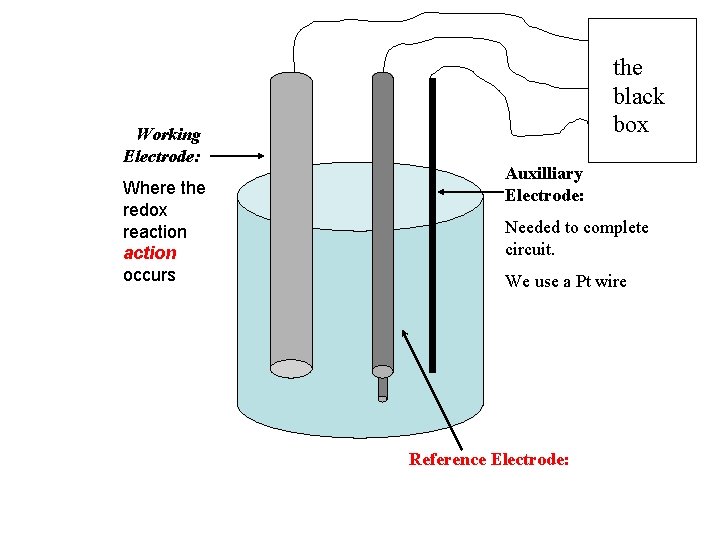
Working Electrode: Where the redox reaction occurs the black box Auxilliary Electrode: Needed to complete circuit. We use a Pt wire Reference Electrode:

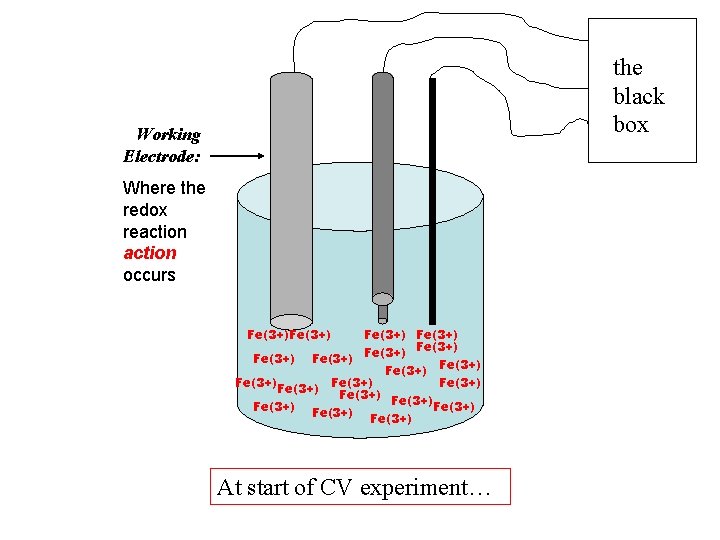
the black box Working Electrode: Where the redox reaction occurs Fe(3+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) At start of CV experiment…

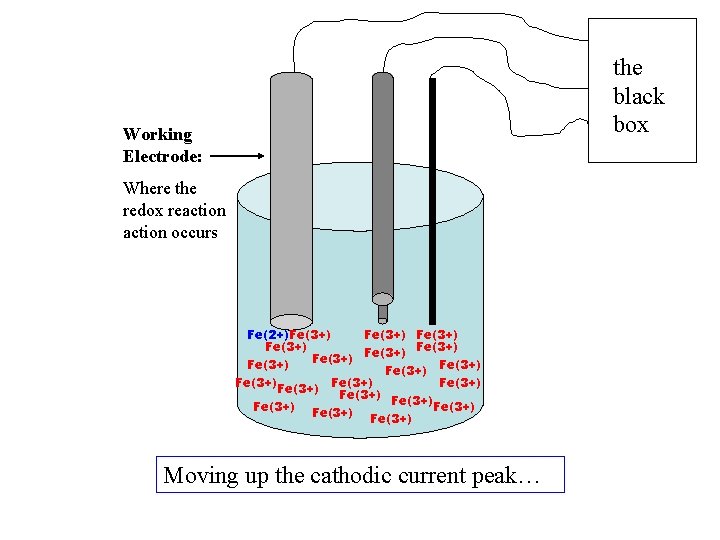
the black box Working Electrode: Where the redox reaction occurs Fe(2+)Fe(3+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) Moving up the cathodic current peak…

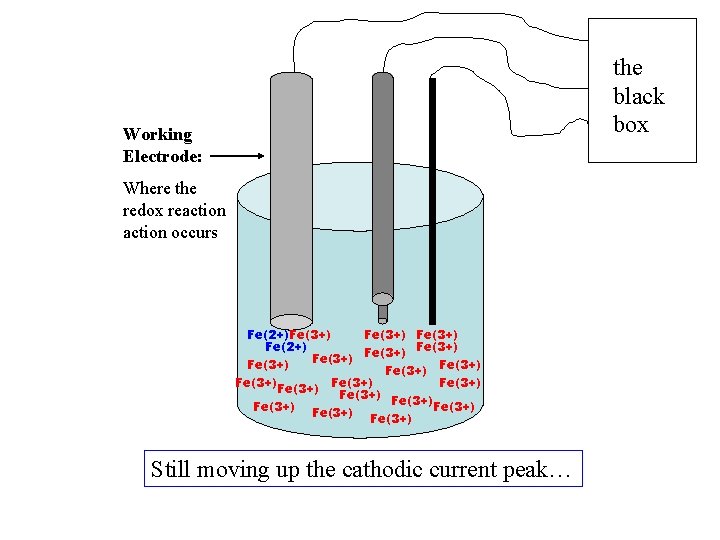
the black box Working Electrode: Where the redox reaction occurs Fe(2+)Fe(3+) Fe(2+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) Still moving up the cathodic current peak…

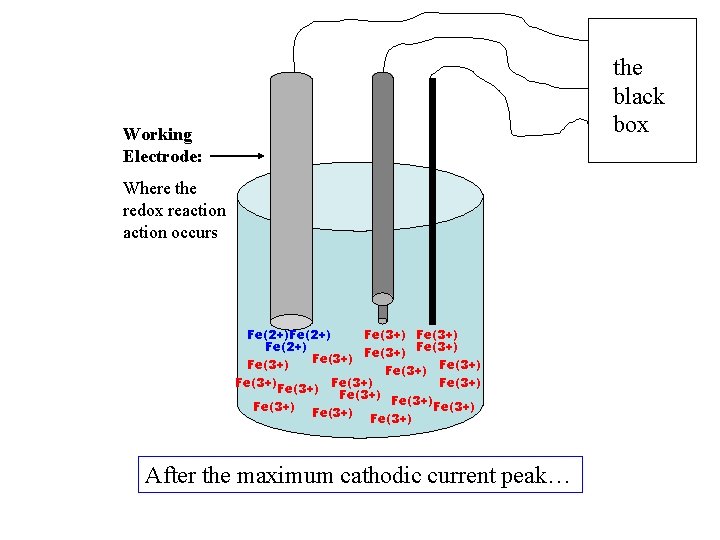
the black box Working Electrode: Where the redox reaction occurs Fe(2+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) After the maximum cathodic current peak…

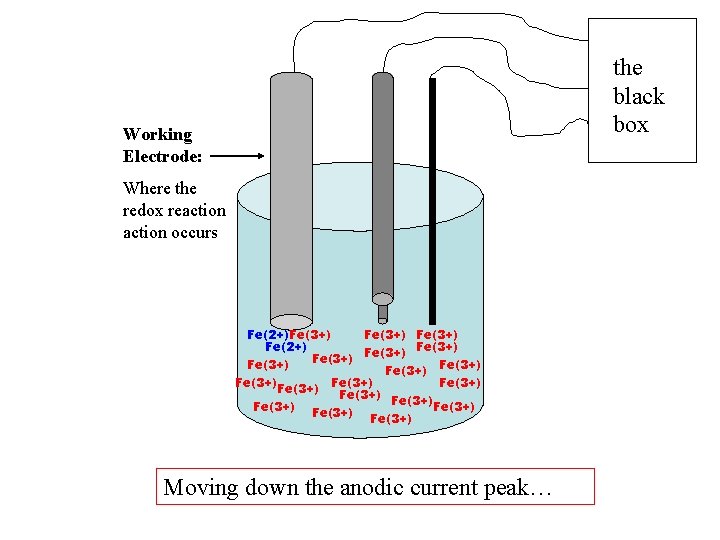
the black box Working Electrode: Where the redox reaction occurs Fe(2+)Fe(3+) Fe(2+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) Moving down the anodic current peak…

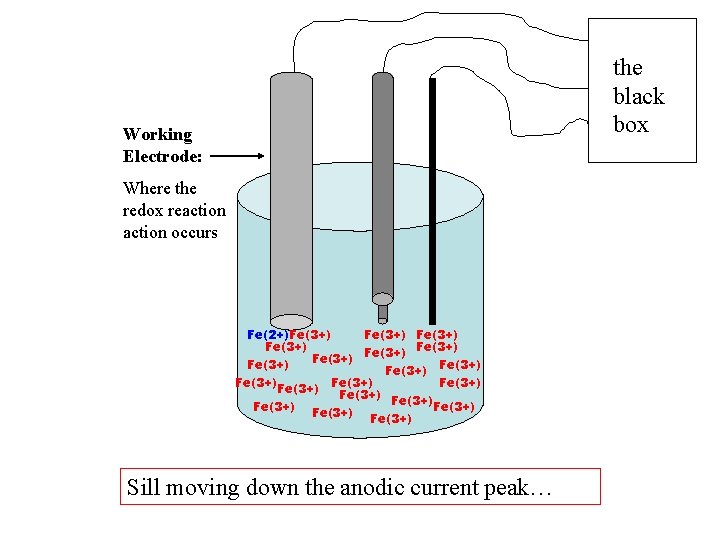
the black box Working Electrode: Where the redox reaction occurs Fe(2+)Fe(3+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) Sill moving down the anodic current peak…

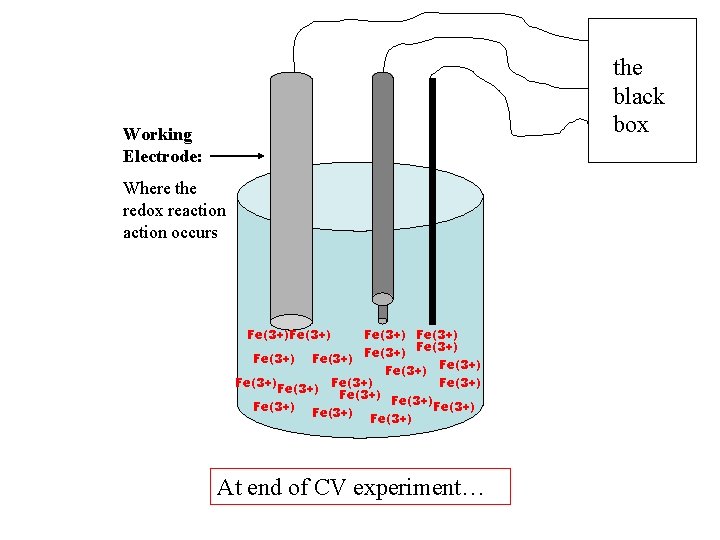
the black box Working Electrode: Where the redox reaction occurs Fe(3+) Fe(3+) Fe(3+) Fe(3+) Fe(3+) At end of CV experiment…

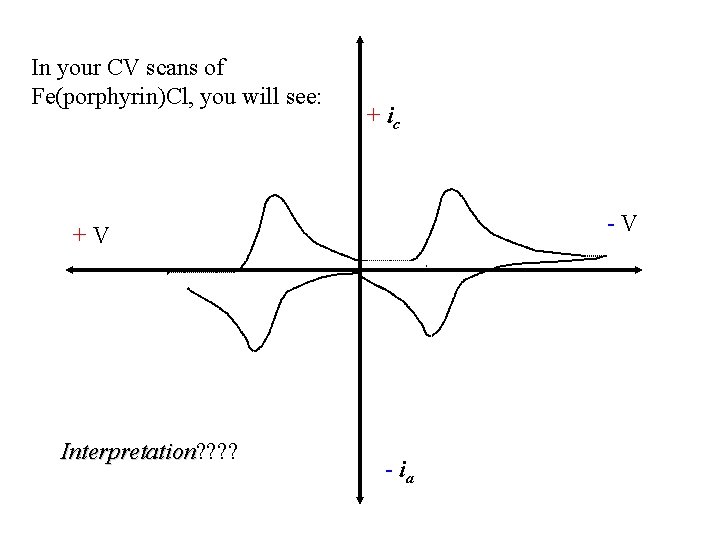
In your CV scans of Fe(porphyrin)Cl, you will see: + ic -V +V Interpretation? ? Interpretation - ia

How is the range of Heme Potentials in Respiration adjusted?

The Question asked: Can changing Heme substituents vary Fe(3+/2+) redcution potentials?






