CURRENT ADVERSE EVENTS REPORTING IN HEMOPHILIA Alfonso Iorio

















- Slides: 25

CURRENT ADVERSE EVENTS REPORTING IN HEMOPHILIA Alfonso Iorio, Mc. Master University, Canada Chair, Data and Demographics Committee, WFH Chair, Canadian Bleeding Disorders Registry PI, Canadian Hemophilia Surveillance Scheme 1

Disclosures for: Alfonso Iorio In compliance with the PIM* policy, WFH requires the following disclosures be made at each presentation CONFLICT DISCLOSURE — IF CONFLICT OF INTEREST EXISTS RESEARCH SUPPORT Mc. Master receives support for research in hemophilia by Bayer, Biogen, Octapharma, Novo-Nordisk, Pfizer, Shire DIRECTOR, OFFICER, EMPLOYEE Data and Demographics committee, WFH SHAREHOLDER HONORARIA ADVISORY COMMITTEE Mc. Master receives support for services in hemophilia by Bayer, Biogen, Octapharma, Novo-Nordisk, Pfizer, Shire CONSULTANT * Postgraduate Institute for Medicine 2

FIRST THEME NAME AGENDA CLICK TO EDIT MASTER TITLE STYLE 1. What is the evidence? 2. What is the use we can make of it? 3. What could everyone role be? 3

Clinical Trial Phases Phase Pre-clinical 1 2 3 4 Study type Animal studies Safety in human volunteers Dosing - efficacy Efficacy comparison with gold standard treatment Post marketing surveillance Pharmacovigilence

WHY RECOMBINANT CONCENTRATES CAUSE MORE COLD AND HEADACHE THAN PLASMA DERIVED ONE? What you get is related to what you put… Pre-licensure trials use a super-powerful magnifying lens to look at one side only of the whole story. . 5

MONITORING SAFETY AFTER LICENSING Post marketing surveillance Formal requirement Reporting schemes EUHASS, CHESS, UKHCDO, France. Coag, Rodin, ATHN National schemes Reporting by doctors, nurses, data managers and patients

MONITORING SAFETY AFTER LICENSING Post marketing surveillance Formal requirement Reporting schemes EUHASS, CHESS, UKHCDO, France. Coag, Rodin, ATHN National schemes Reporting by doctors, nurses, data managers and patients

POST MARKETING SURVEILLANCE

POST MARKETING SURVEILLANCE After RODIN publication, I (as an educated citizen // cumbersome open access ) have accessed the Health Canada database: over 10 years, 6. 7 more inhibitor with kogenate (27 vs 4)

POST MARKETING SURVEILLANCE

POST-HOC ANALYSIS OF POST-MARKETING SURVEILLANCE STUDIES § Iorio A et al. Patient data meta-analysis of Post-Authorization Safety Surveillance (PASS) studies of haemophilia A patients treated with r. AHF-PFM. Haemophilia. 20(6), 777– 83 (2014). § Romanov V et al. Evaluation of Safety and Effectiveness of factor VIII treatment in Hemophilia A patients with low titer inhibitors or a personal history of inhibitor. Thromb. Haemost. 114, 56– 64 (2015). § Bayesian approach to the assessment of the population specific risk of inhibitors in Hemophilia A patients: a case study. Journal of Blood Medicine, accepted 11

MONITORING SAFETY AFTER LICENSING Post marketing surveillance Formal requirement Reporting schemes EUHASS, CHESS, UKHCDO, France. Coag, Rodin, ATHN National schemes Reporting by doctors, nurses, data managers and patients

MONITORING SAFETY AFTER LICENSING EUHASS Nat 1 CHESS UKHCDO Nat 2 RODIN France. C ATHN

MONITORING SAFETY AFTER LICENSING Effort EUHASS Nat 1 By program characteristics CHESS UKHCDO RODIN France. C Nat 2 Size ATHN

MONITORING SAFETY AFTER LICENSING Richness EUHASS Nat 1 By data characteristics CHESS UKHCDO RODIN France. C ATHN Nat 2 Coverage

MONITORING SAFETY AFTER LICENSING

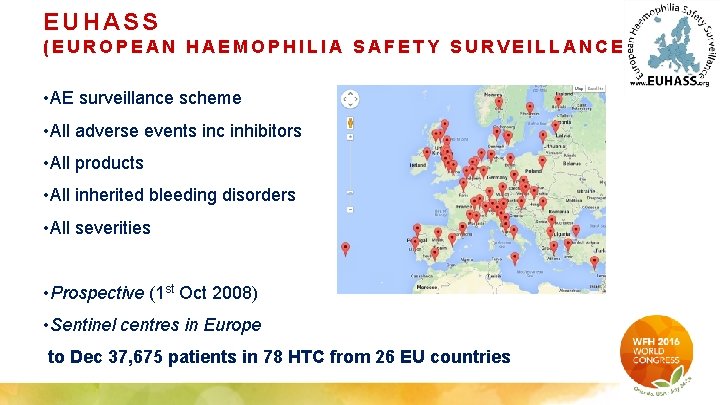
EUHASS (EUROPEAN HAEMOPHILIA SAFETY SURVEILLANCE) • AE surveillance scheme • All adverse events inc inhibitors • All products • All inherited bleeding disorders • All severities • Prospective (1 st Oct 2008) • Sentinel centres in Europe to Dec 37, 675 patients in 78 HTC from 26 EU countries

CONCENTRATES USED JAN 2014 - DEC 2014 69 DIFFERENT PRODUCTS Recombinant • Advate, 2580 • Kogenate/Helixate, 2578 • Re. Facto AF 1695 • Bene. Fix, 1187 18

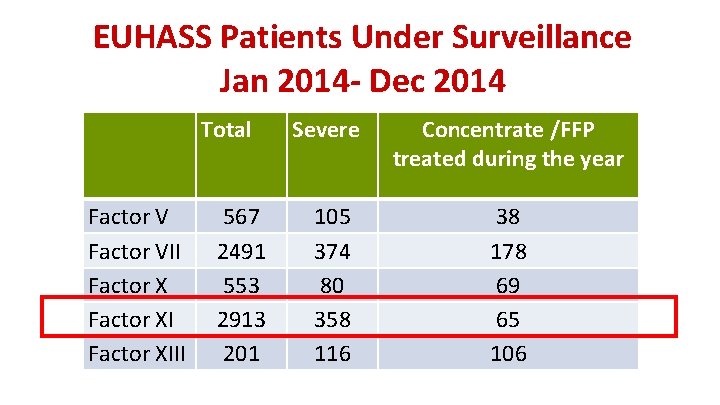
EUHASS Patients Under Surveillance Jan 2014 - Dec 2014 Total Factor VII Factor XIII 567 2491 553 2913 201 Severe Concentrate /FFP treated during the year 105 374 80 358 116 38 178 69 65 106

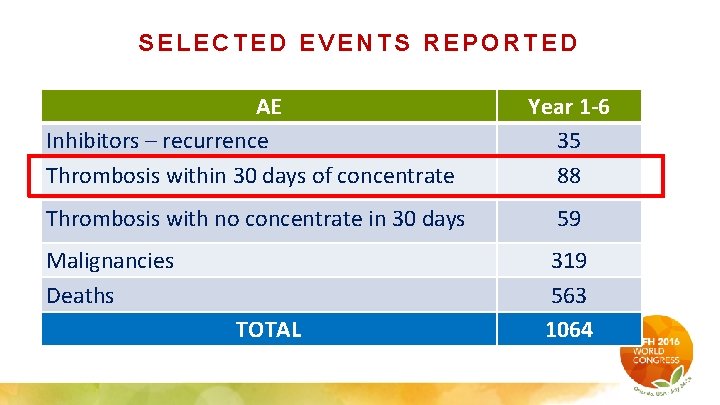
SELECTED EVENTS REPORTED AE Inhibitors – recurrence Thrombosis within 30 days of concentrate Year 1 -6 35 88 Thrombosis with no concentrate in 30 days 59 Malignancies Deaths TOTAL 319 563 1064

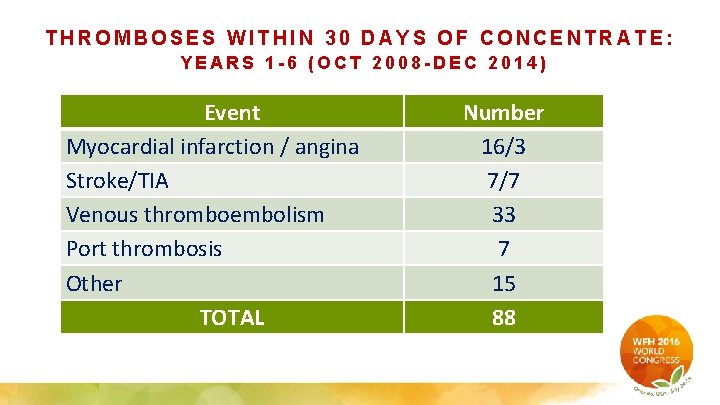
THROMBOSES WITHIN 30 DAYS OF CONCENTRATE: YEARS 1 -6 (OCT 2008 -DEC 2014) Event Myocardial infarction / angina Stroke/TIA Venous thromboembolism Port thrombosis Other TOTAL Number 16/3 7/7 33 7 15 88

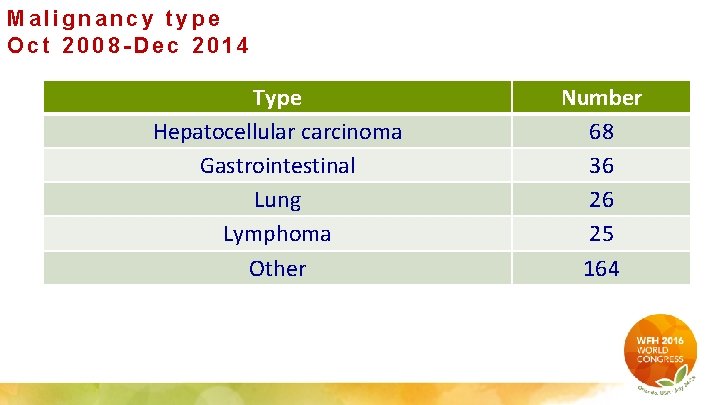
Malignancy type Oct 2008 -Dec 2014 Type Hepatocellular carcinoma Gastrointestinal Lung Lymphoma Other Number 68 36 26 25 164

LARGE SCALE STUDIES: STRENGTHS Large numbers Quicker identification of danger signals No selection bias All products monitored at same time Prospective Not industry led Long duration Less costly than industry studies Earlier results Collaboration between participants for other projects

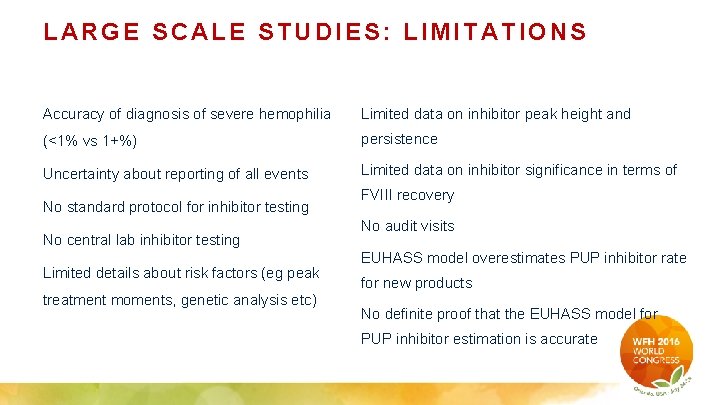
LARGE SCALE STUDIES: LIMITATIONS Accuracy of diagnosis of severe hemophilia Limited data on inhibitor peak height and (<1% vs 1+%) persistence Uncertainty about reporting of all events Limited data on inhibitor significance in terms of No standard protocol for inhibitor testing No central lab inhibitor testing Limited details about risk factors (eg peak treatment moments, genetic analysis etc) FVIII recovery No audit visits EUHASS model overestimates PUP inhibitor rate for new products No definite proof that the EUHASS model for PUP inhibitor estimation is accurate

ACKNOWLEDGEMENTS Mike Makris for the slides on EUHASS/CHESS network collaborators Slides at http: //hemophilia. mcmaster. ca
 Novartis adverse event reporting
Novartis adverse event reporting Ahca adverse incident reporting
Ahca adverse incident reporting Adverse events in hospital
Adverse events in hospital Ir adverse event
Ir adverse event Adverse events in hospital
Adverse events in hospital Adverse events following immunization (aefi) course answers
Adverse events following immunization (aefi) course answers Events after the reporting date
Events after the reporting date Mutually exclusive events vs not mutually exclusive events
Mutually exclusive events vs not mutually exclusive events Current events presentation
Current events presentation Weekly current events quiz
Weekly current events quiz Middle ages jeopardy
Middle ages jeopardy What is name of
What is name of Delta to wye conversion balanced
Delta to wye conversion balanced Hazard based safety engineering
Hazard based safety engineering Line current and phase current
Line current and phase current Drift current
Drift current Slideplayer
Slideplayer Kcl mesh analysis
Kcl mesh analysis Drift current and diffusion current
Drift current and diffusion current Energy band diagram of pn junction diode
Energy band diagram of pn junction diode Diffusion current formula
Diffusion current formula The jfet always operates with
The jfet always operates with Chapter 3 shielded metal arc equipment setup and operation
Chapter 3 shielded metal arc equipment setup and operation Y connected generator
Y connected generator Line current and phase current
Line current and phase current Types of hemophilia
Types of hemophilia