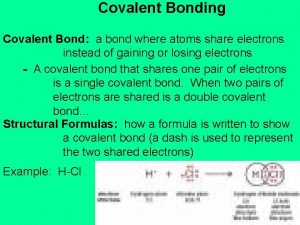
Covalent Bonding What is a covalent bond Covalent

Covalent Bonding • What is a covalent bond?
Covalent Bonding • What is a covalent bond? • How is a covalent bond different from an ionic bond?
Covalent Bonding • What is a covalent bond? • How is a covalent bond different from an ionic bond? • How is bond length and bond energy related to the stability of a covalent bond?

Covalent Bonding • What is a covalent bond? • How is a covalent bond different from an ionic bond? • How is bond length and bond energy related to the stability of a covalent bond? • What is the difference between nonpolar covalent bonding and polar covalent bonding.
Covalent Bonding • What is a covalent bond? • How is a covalent bond different from an ionic bond? • How is bond length and bond energy related to the stability of a covalent bond? • What is the difference between nonpolar covalent bonding and polar covalent bonding. • Electronegativity vs Bond Type
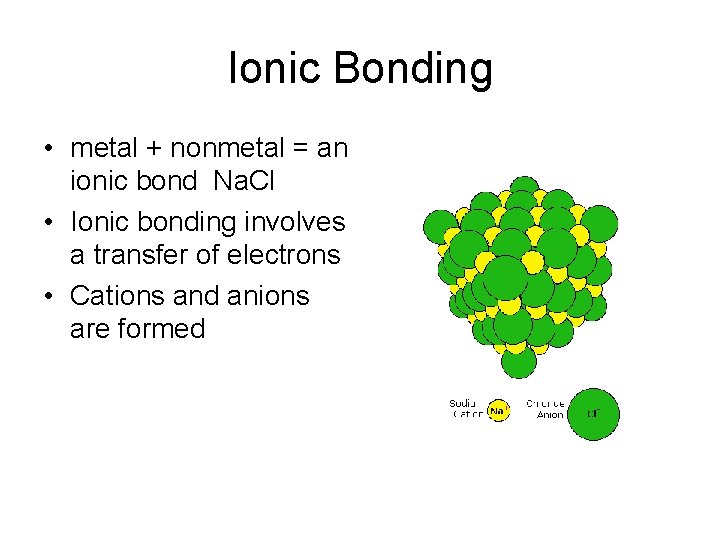
Ionic Bonding • metal + nonmetal = an ionic bond Na. Cl • Ionic bonding involves a transfer of electrons • Cations and anions are formed
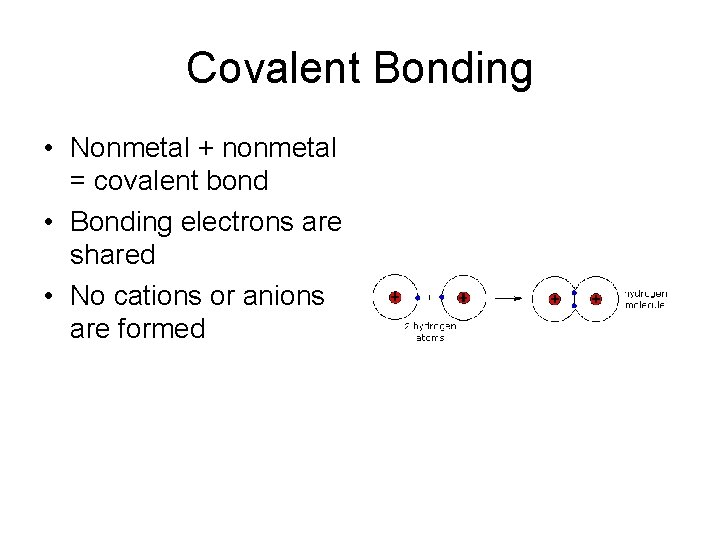
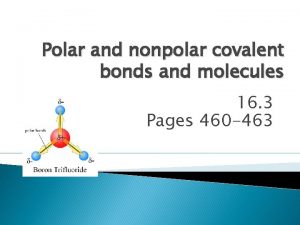
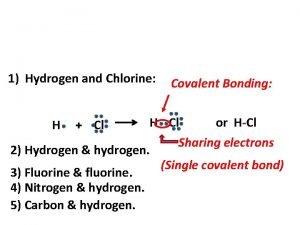
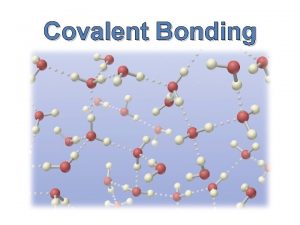
Covalent Bonding • Nonmetal + nonmetal = covalent bond • Bonding electrons are shared • No cations or anions are formed
Covalent Compounds • Like ionic compounds covalent compounds are most stable when they follow the octet rule.
Covalent Bonding • What forces hold a covalent bond together?
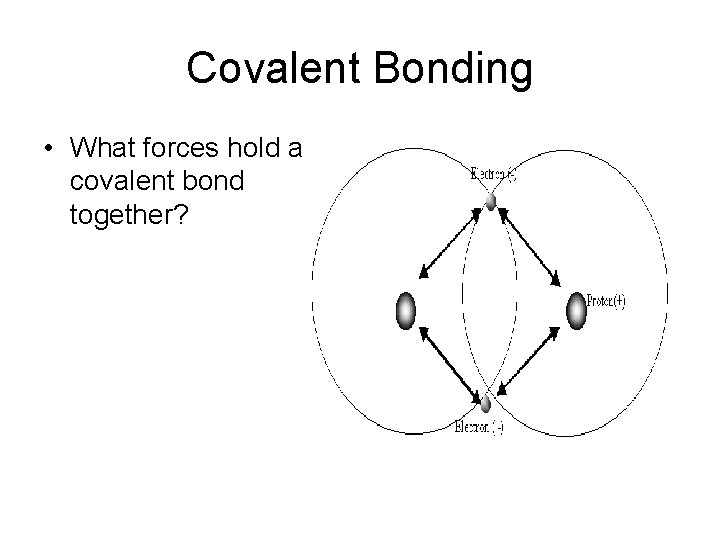
Covalent Bonding • What forces hold a covalent bond together?
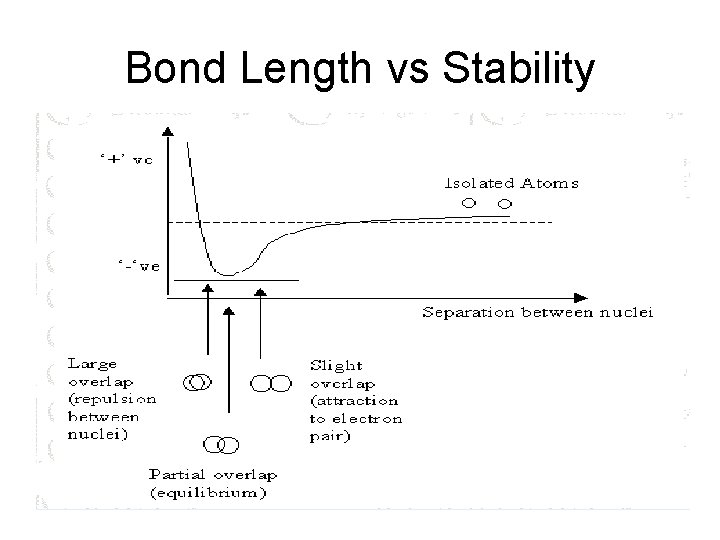
Bond Length vs Stability

Bond Length vs Stability • In general, the shorter the bond length the more stable the bond. • Bond stability can be measure by how much energy it takes to break the bond.
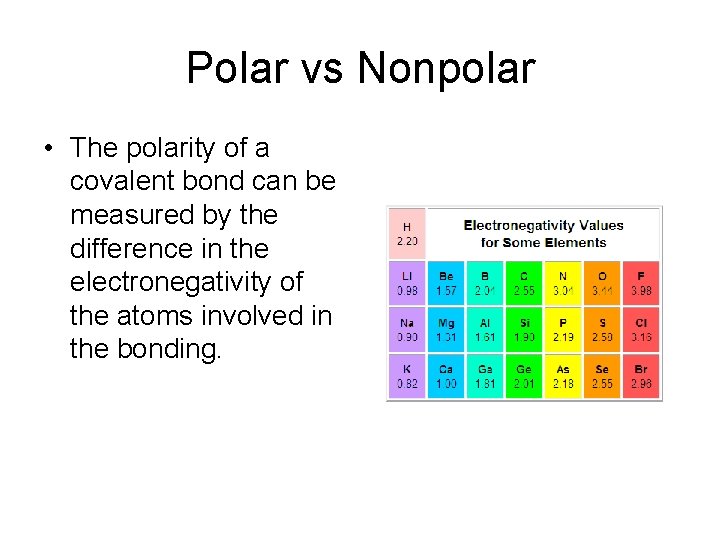
Polar vs Nonpolar • The polarity of a covalent bond can be measured by the difference in the electronegativity of the atoms involved in the bonding.
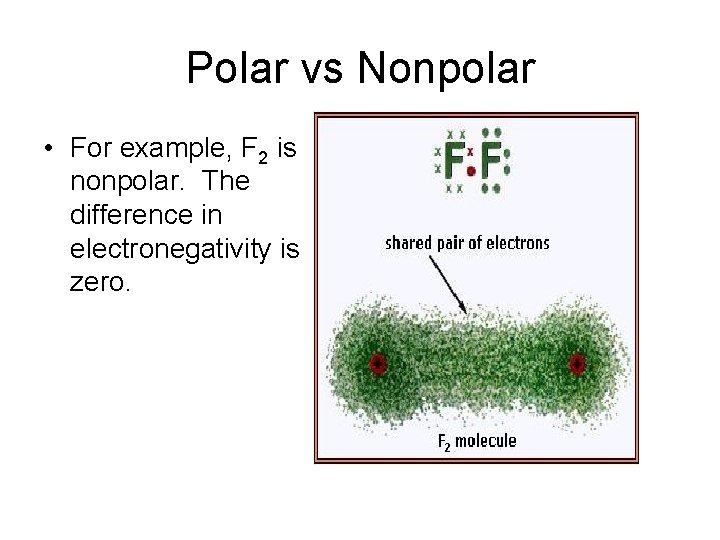
Polar vs Nonpolar • For example, F 2 is nonpolar. The difference in electronegativity is zero.
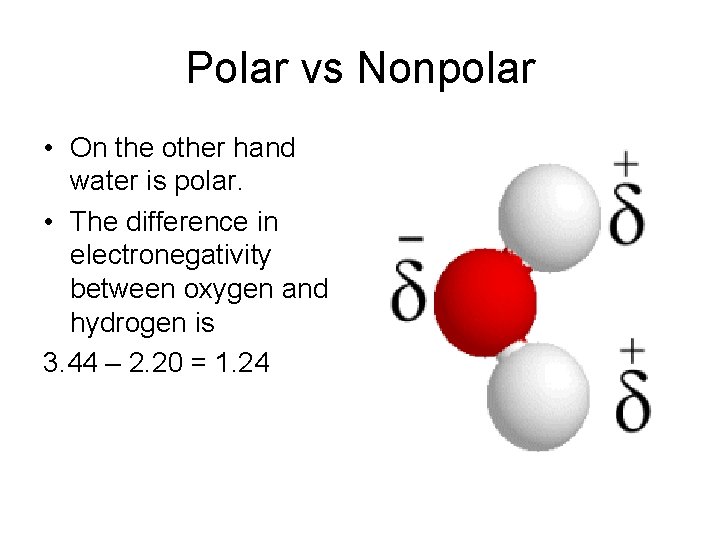
Polar vs Nonpolar • On the other hand water is polar. • The difference in electronegativity between oxygen and hydrogen is 3. 44 – 2. 20 = 1. 24
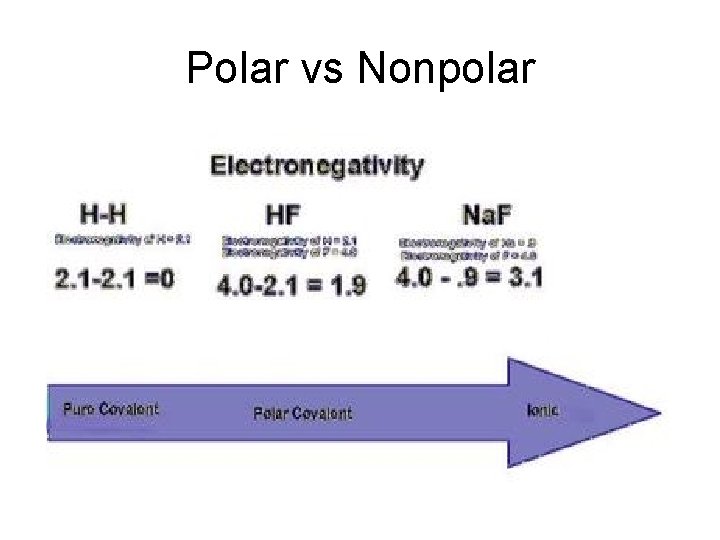
Polar vs Nonpolar

Polar vs Nonpolar • A difference in electronegativity of 0. 0 to 0. 5 is considered to be nonpolar. • A difference in electronegativity of 0. 5 to 2. 1 is considered to be polar. • Anything over 2. 1 is considered to be ionic.
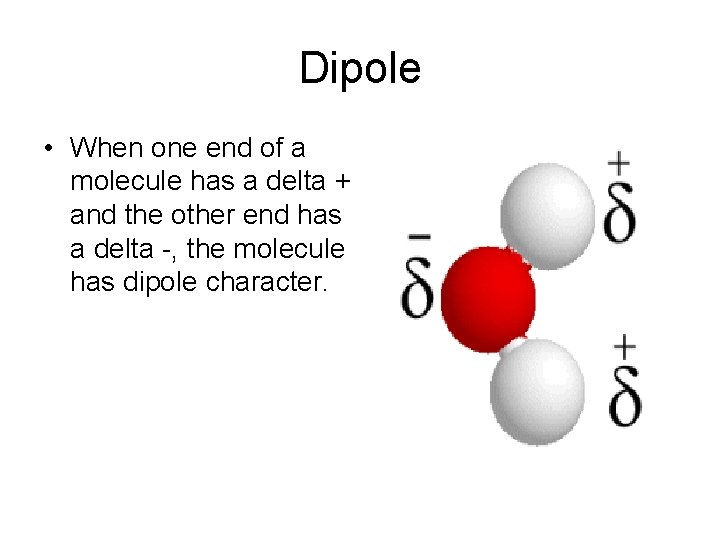
Dipole • When one end of a molecule has a delta + and the other end has a delta -, the molecule has dipole character.

Like Dissolves Like • Water is a dipole therefore ionic or polar substances will dissolve in water. Nonpolar substances will not dissolve in water. • Benzene is a nonpolar molecule. Nonpolar substances such as oil or gasoline will dissolve in benzene.
- Slides: 19