Column Chromatography 1903 Mikhail Tswett a Russian scientist









- Slides: 15

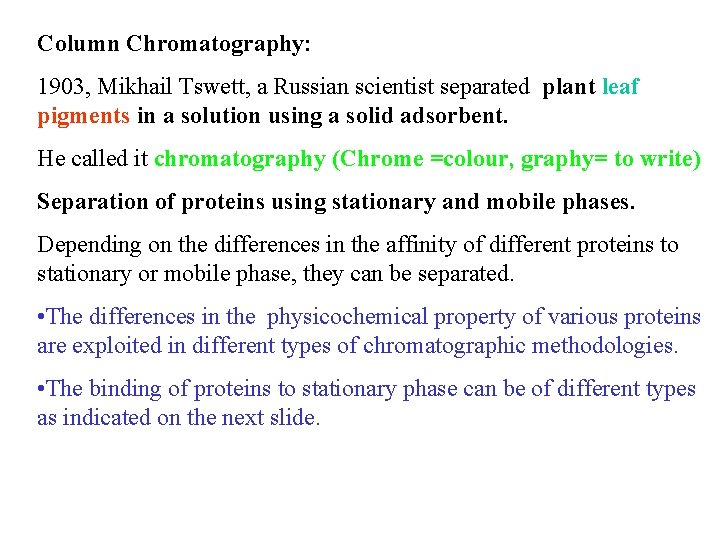
Column Chromatography: 1903, Mikhail Tswett, a Russian scientist separated plant leaf pigments in a solution using a solid adsorbent. He called it chromatography (Chrome =colour, graphy= to write) Separation of proteins using stationary and mobile phases. Depending on the differences in the affinity of different proteins to stationary or mobile phase, they can be separated. • The differences in the physicochemical property of various proteins are exploited in different types of chromatographic methodologies. • The binding of proteins to stationary phase can be of different types as indicated on the next slide.

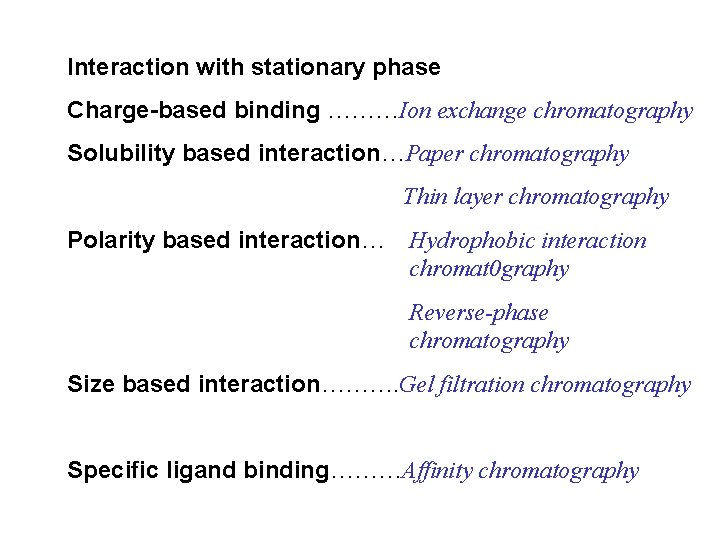
Interaction with stationary phase Charge-based binding ………Ion exchange chromatography Solubility based interaction…Paper chromatography Thin layer chromatography Polarity based interaction… Hydrophobic interaction chromat 0 graphy Reverse-phase chromatography Size based interaction………. Gel filtration chromatography Specific ligand binding………Affinity chromatography


Ion exchange chromatography: • This technique exploites the differences in the sign (negative or positive) and magnitude of net electric charges on proteins at a given p. H. • The stationary phase in chromatography is usually made of synthetic polymers (chemically innert species) covalently linked with charged species. • Matrix with –ive charge = can bind electro-statically to +ive charge species, which can be replaced by an other +ive species. This matrix is referred as Cation exchanger. Similarly, Matrix with positive charge are anion exchangers.



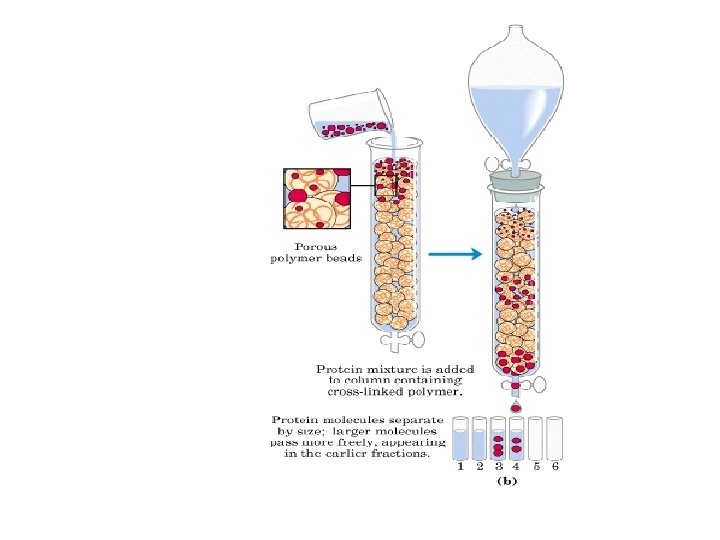
Gel filtration chromatography: Also called size exclusion chromatography • The stationary phase is made up of cross-linked polymers with pores of selected sizes. • Large proteins do not get stuck with the matrix and migrate faster, whereas smaller proteins keep getting stuck with the pores and migrate slowly. This technique is very useful in studies involving protein-protein interaction and protein size determination.



Affinity chromatography: This is very efficient chromatographic method to purify proteins in relatively short time. The main requirement is that a known ligand or specific antibody should be known for the protein being purified. The ligand (antibody or peptides with high affinity for the protein) are covalently linked to a resin or matrix. This ligand-cojugated matrix is used at stationary phase in the column. Protein extracts containing all the proteins are passed through the column in a low salt buffer. The culumn is washed with low salt buffer so that unbound proteins are washed out. Salt concentration is slowly increased, and fractions are collected. At higher salt concentration, the binding with ligand is destabilized and it is eluted from the column.





 Mikhail tswett chromatography
Mikhail tswett chromatography Michael tswett chromatography
Michael tswett chromatography Mikhail tswett
Mikhail tswett Rumus waktu retensi
Rumus waktu retensi Mikhail tswett
Mikhail tswett Chromatography principle
Chromatography principle Mikhail tswett cromatografia
Mikhail tswett cromatografia Cyperm
Cyperm M.s. tswett and the invention of chromatography
M.s. tswett and the invention of chromatography Rumus waktu retensi
Rumus waktu retensi Advantages of chromatography
Advantages of chromatography Dry column vacuum chromatography
Dry column vacuum chromatography General theory of chromatography
General theory of chromatography Rate theory and plate theory
Rate theory and plate theory Paper chromatography youtube
Paper chromatography youtube 56fe isotope
56fe isotope