Closure of Iatrogenic Atrial Septal Defects Following Transcatheter





- Slides: 20

Closure of Iatrogenic Atrial Septal Defects Following Transcatheter Mitral Valve Repair – The Randomized MITHRAS Trial Philipp Lurz, Matthias Unterhuber, Karl-Philipp Rommel, Karl-Patrik Kresoja, Tobias Kister, Christian Besler, Karl Fengler, Marcus Sandri, Ingo Daehnert, Maximilian von Roeder, Stephan Blazek, Holger Thiele Heart Center Leipzig at University of Leipzig

Disclosure Statement of Financial Interest Speaker’s Name: Philipp Lurz Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below Affiliation/Financial Relationship Company Institutional Grant/Research Support Re. Cor, Occlutech, Edwards Lifesciences Consulting Fees to Institution Abbott, Re. Cor, Occlutech, Edwards Lifesciences, Medtronic Personal Fees None Major Stock Shareholder/Equity None Royalty Income None Ownership/Founder None Intellectual Property Rights None

Background • Transcatheter therapies for mitral regurgitation (TMVR) require transseptal access to left atrium. • Persistent iatrogenic atrial septal defect (i. ASD) in 24 -50% of patients. • i. ASD with left-to-right shunting associated with right heart volume overload and mortality. • In contrast, creation of an i. ASD linked to improved hemodynamics and is investigated in large-scale clinical trials in patients with heart failure

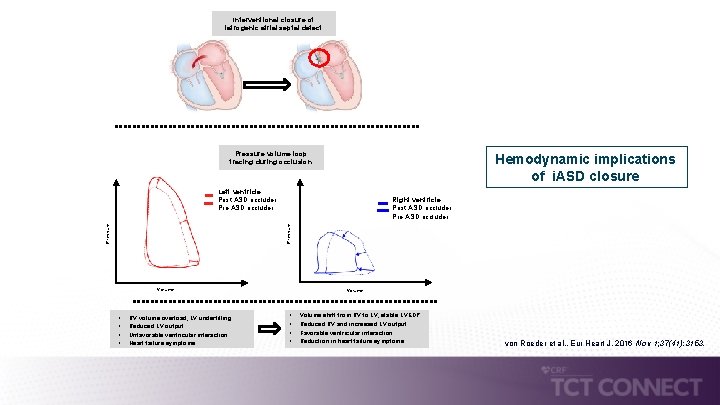
Interventional closure of iatrogenic atrial septal defect Pressure volume loop tracing during occlusion Hemodynamic implications of i. ASD closure Left ventricle Post ASD occluder Pressure Right ventricle Post ASD occluder Pre ASD occluder Volume • • RV volume overload, LV underfilling Reduced LV output Unfavorable ventricular interaction Heart failure symptoms Volume • • Volume shift from RV to LV, stable LVEDP Reduced RV and incresaed LV output Favorable ventricular interaction Reduction in heart failure symptoms von Roeder et al. , Eur Heart J, 2016 Nov 1; 37(41): 3153.

Objective Interventional closure Conservative treatment Persistent i. ASD Given the controversy and lack of recommendations in the setting of a persistent i. ASD with relevant left-to-right shunting following TMVR, we investigated whether the closure of a TMVR induced i. ASD is superior to conservative treatment in a randomized controlled trial.

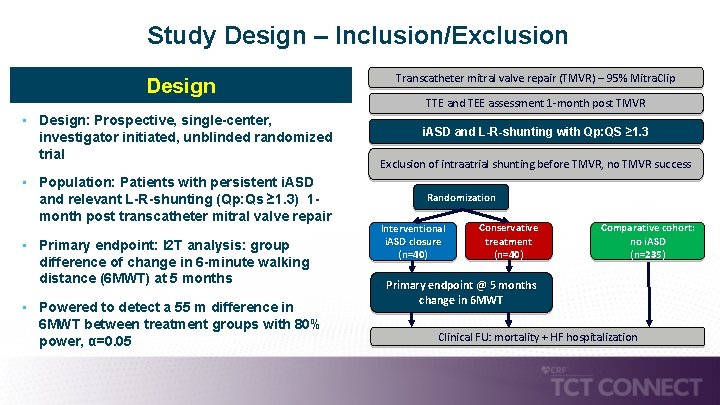
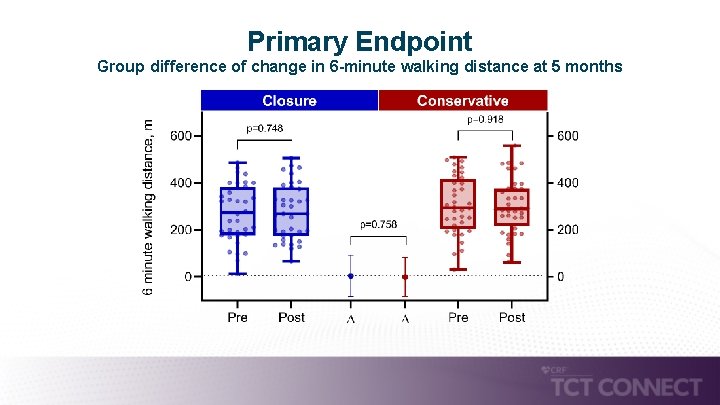
Study Design – Inclusion/Exclusion Design • Design: Prospective, single-center, investigator initiated, unblinded randomized trial • Population: Patients with persistent i. ASD and relevant L-R-shunting (Qp: Qs ≥ 1. 3) 1 - month post transcatheter mitral valve repair • Primary endpoint: I 2 T analysis: group difference of change in 6 -minute walking distance (6 MWT) at 5 months • Powered to detect a 55 m difference in 6 MWT between treatment groups with 80% power, α=0. 05 Transcatheter mitral valve repair (TMVR) – 95% Mitra. Clip TTE and TEE assessment 1 -month post TMVR i. ASD and L-R-shunting with Qp: QS ≥ 1. 3 Exclusion of intraatrial shunting before TMVR, no TMVR success Randomization Interventional i. ASD closure (n=40) Conservative treatment (n=40) Comparative cohort: no i. ASD (n=235) Primary endpoint @ 5 months change in 6 MWT Clinical FU: mortality + HF hospitalization

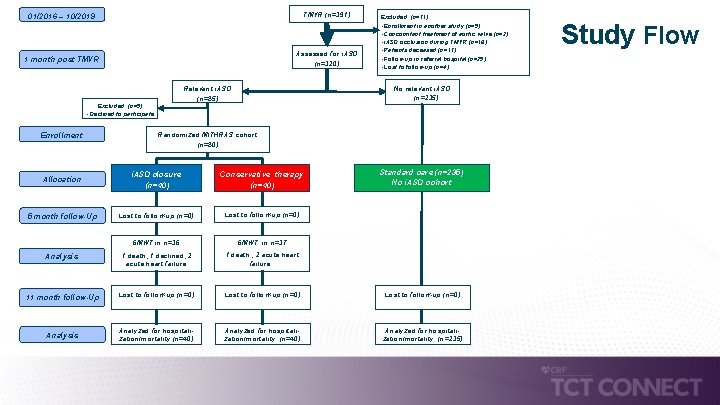
01/2016 – 10/2019 TMVR (n=391) 1 month post TMVR Assessed for i. ASD (n=320) No relevant i. ASD (n=235) Relevant i. ASD (n=85) Excluded (n=5) • Declined to participate Enrollment Excluded (n=71) • Enrollment in another study (n=5) • Concomitant treatment of aortic valve (n=2) • i. ASD occlusion during TMVR (n=18) • Patients deceased (n=17) • Follow-up in referral hospital (n=25) • Lost to follow-up (n=4) Randomized MITHRAS cohort (n=80) Standard care (n=235) No i. ASD cohort i. ASD closure (n=40) Conservative therapy (n=40) Lost to follow-up (n=0) 6 MWT in n=36 6 MWT in n=37 Analysis 1 death, 1 declined, 2 acute heart failure 1 death , 2 acute heart failure 11 month follow-Up Lost to follow-up (n=0) Analysis Analyzed for hospitalization/mortality (n=40) Analyzed for hospitalization/mortality (n=235) Allocation 5 month follow-Up Study Flow

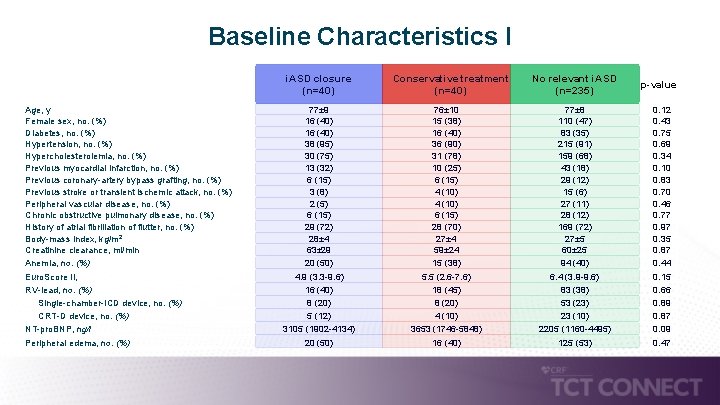
Baseline Characteristics I i. ASD closure (n=40) Age, y Female sex, no. (%) Diabetes, no. (%) Hypertension, no. (%) Hypercholesterolemia, no. (%) Previous myocardial infarction, no. (%) Previous coronary-artery bypass grafting, no. (%) Previous stroke or transient ischemic attack, no. (%) Peripheral vascular disease, no. (%) Chronic obstructive pulmonary disease, no. (%) History of atrial fibrillation of flutter, no. (%) Body-mass index, kg/m 2 Creatinine clearance, ml/min Anemia, no. (%) Euro. Score II, RV-lead, no. (%) Single-chamber-ICD device, no. (%) CRT-D device, no. (%) NT-pro. BNP, ng/l Peripheral edema, no. (%) 77± 9 16 (40) 38 (95) 30 (75) 13 (32) 6 (15) 3 (8) 2 (5) 6 (15) 29 (72) 28± 4 63± 29 20 (50) 4. 9 (3. 3 -9. 6) 16 (40) 8 (20) 5 (12) 3105 (1902 -4134) 20 (50) Conservative treatment (n=40) 76± 10 15 (38) 16 (40) 36 (90) 31 (78) 10 (25) 6 (15) 4 (10) 6 (15) 28 (70) 27± 4 59± 24 15 (38) 5. 5 (2. 6 -7. 6) 18 (45) 8 (20) 4 (10) 3653 (1746 -5848) 16 (40) No relevant i. ASD (n=235) 77± 8 110 (47) 83 (35) 215 (91) 159 (68) 43 (18) 29 (12) 15 (6) 27 (11) 28 (12) 169 (72) 27± 5 60± 25 94 (40) 6. 4 (3. 9 -9. 6) 83 (38) 53 (23) 23 (10) 2205 (1160 -4495) 125 (53) p-value 0. 12 0. 43 0. 75 0. 69 0. 34 0. 10 0. 83 0. 70 0. 46 0. 77 0. 97 0. 35 0. 87 0. 44 0. 15 0. 66 0. 89 0. 87 0. 09 0. 47

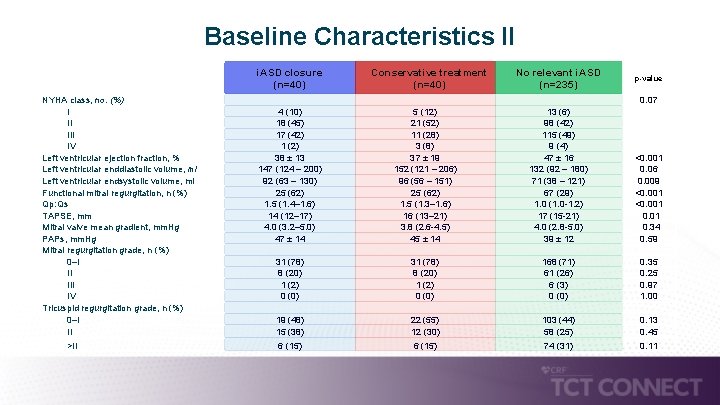
Baseline Characteristics II i. ASD closure (n=40) NYHA class, no. (%) I II IV Left ventricular ejection fraction, % Left ventricular enddiastolic volume, ml Left ventricular endsystolic volume, ml Functional mitral regurgitation, n (%) Qp: Qs TAPSE, mm Mitral valve mean gradient, mm. Hg PAPs, mm. Hg Mitral regurgitation grade, n (%) 0–I II IV Tricuspid regurgitation grade, n (%) 0–I II >II 4 (10) 18 (45) 17 (42) 1 (2) 38 ± 13 147 (124 – 200) 92 (63 – 130) 25 (62) 1. 5 (1. 4– 1. 6) 14 (12– 17) 4. 0 (3. 2– 5. 0) 47 ± 14 31 (78) 8 (20) 1 (2) 0 (0) 19 (48) 15 (38) 6 (15) Conservative treatment (n=40) 5 (12) 21 (52) 11 (28) 3 (8) 37 ± 19 152 (121 – 206) 96 (56 – 151) 25 (62) 1. 5 (1. 3– 1. 6) 16 (13– 21) 3. 8 (2. 6 -4. 5) 45 ± 14 31 (78) 8 (20) 1 (2) 0 (0) 22 (55) 12 (30) 6 (15) No relevant i. ASD (n=235) p-value 13 (6) 98 (42) 115 (49) 9 (4) 47 ± 16 132 (92 – 180) 71 (38 – 121) 67 (29) 1. 0 (1. 0 -1. 2) 17 (15 -21) 4. 0 (2. 8 -5. 0) 39 ± 12 168 (71) 61 (26) 6 (3) 0 (0) 103 (44) 58 (25) 74 (31) 0. 07 <0. 001 0. 06 0. 009 <0. 001 0. 34 0. 59 0. 35 0. 25 0. 97 1. 00 0. 13 0. 45 0. 11

Primary Endpoint Group difference of change in 6 -minute walking distance at 5 months

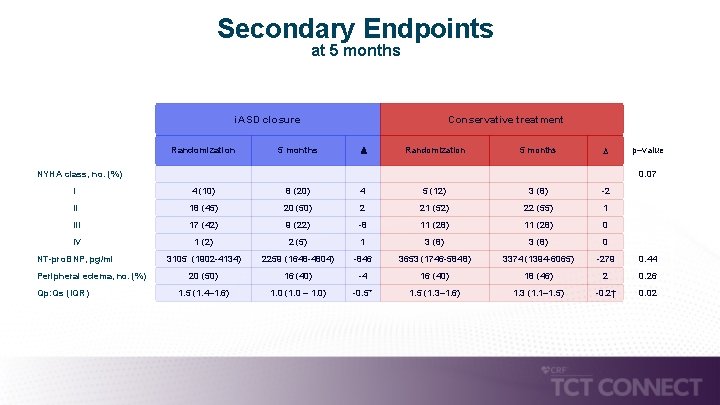
Secondary Endpoints at 5 months i. ASD closure Conservative treatment Randomization 5 months p–value 0. 07 I 4 (10) 8 (20) 4 5 (12) 3 (8) -2 II 18 (45) 20 (50) 2 21 (52) 22 (55) 1 III 17 (42) 9 (22) -8 11 (28) 0 IV 1 (2) 2 (5) 1 3 (8) 0 NT-pro. BNP, pg/ml 3105 (1902 -4134) 2259 (1648 -4804) -846 3653 (1746 -5848) 3374 (1394 -6065) -279 0. 44 20 (50) 16 (40) -4 16 (40) 18 (46) 2 0. 26 1. 5 (1. 4– 1. 6) 1. 0 (1. 0 – 1. 0) -0. 5* 1. 5 (1. 3– 1. 6) 1. 3 (1. 1– 1. 5) -0. 2† 0. 02 NYHA class, no. (%) Peripheral edema, no. (%) Qp: Qs (IQR)

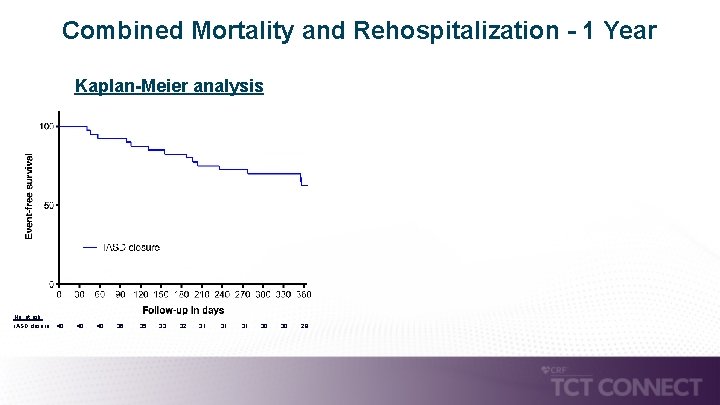
Combined Mortality and Rehospitalization - 1 Year Kaplan-Meier analysis No. at risk: i. ASD closure: 40 40 36 35 33 32 31 31 30 28

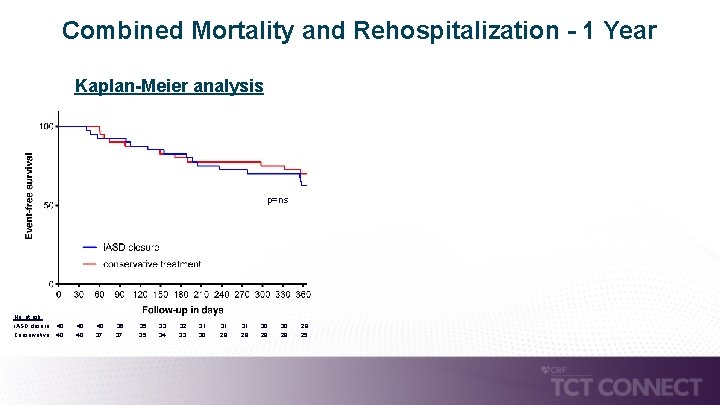
Combined Mortality and Rehospitalization - 1 Year Kaplan-Meier analysis p=ns No. at risk: i. ASD closure: 40 40 36 35 33 32 31 Conservative: 40 37 35 34 33 30 29 31 30 28 29 28 25

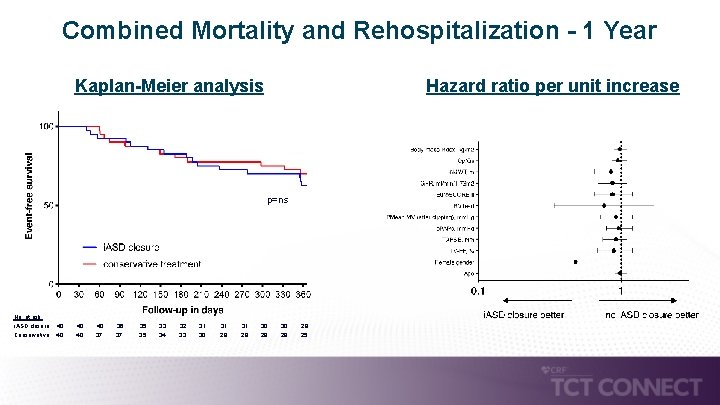
Combined Mortality and Rehospitalization - 1 Year Kaplan-Meier analysis Hazard ratio per unit increase p=ns No. at risk: i. ASD closure: 40 40 36 35 33 32 31 Conservative: 40 37 35 34 33 30 29 31 30 28 29 28 25

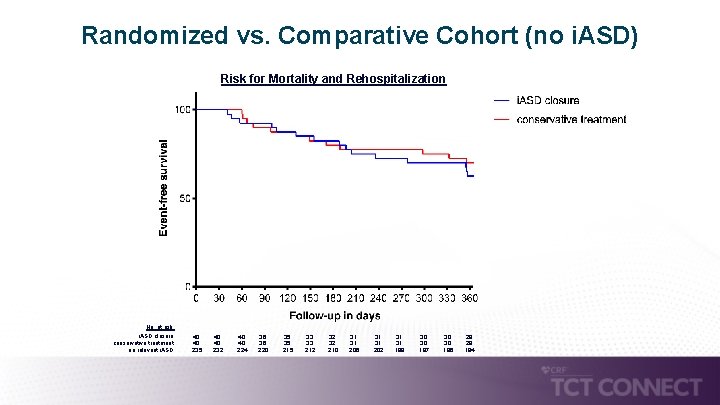
Randomized vs. Comparative Cohort (no i. ASD) Risk for Mortality and Rehospitalization No. at risk: i. ASD closure: conservative treatment: no relevant i. ASD: 40 40 235 40 40 232 40 40 224 36 36 220 35 35 215 33 33 212 32 32 210 31 31 206 31 31 202 31 31 199 30 30 197 30 30 196 28 28 194

Randomized vs. Comparative Cohort (no i. ASD) Risk for Mortality and Rehospitalization No. at risk: i. ASD closure: conservative treatment: no relevant i. ASD: 40 40 235 40 40 232 40 40 224 36 36 220 35 35 215 33 33 212 32 32 210 31 31 206 31 31 202 31 31 199 30 30 197 30 30 196 28 28 194

Limitations • Single-center and relatively small number of patients. • Randomization and analysis not stratified according to etiology of mitral regurgitation. • Results might vary depending on different degrees of RV dysfunction and volume overload as well as different left sided filling pressures. • Results might vary in specific subsets of patients. • Although mean Qp: Qs was 1. 5, shunting volumes might have been to small to detect benefits of closure. • L-R shunting across i. ASD can decrease over time without interventional closure inclusion and closure of i. ASD might have been too early to differentiate treatment benefits.

Summary • Interventional closure of i. ASD 1 -month post transcatheter mitral valve repair was not superior to conservative treatment with regards to the primary endpoint 6 -minute walking distance. • The results are corroborated by no difference in secondary endpoints such as heart failure symptoms or hospitalization and survival. • The presence of an i. ASD is associated with a higher rate of HF hospitalization irrespective of its management when compared to patients without relevant i. ASD following TMVR.

Conclusions • The presence of an i. ASD following transcatheter mitral valve interventions might be a prognostically relevant surrogate, but not necessarily causative for inferior outcomes. • Patients with a persistent i. ASD and relevant left-to-right shunting appear at higher risk with potential implications for surveillance and management other than interventional i. ASD closure. • i. ASD closure remains an individualized decision on case-by-case basis with no evidence in support of general recommendation to close. Results of primary endpoint simultaneously published in Circulation

Thank You philipp. lurz@medizin. uni-Leipzig. de
 Atrial septal defect
Atrial septal defect Coarctatie de aorta definitie
Coarctatie de aorta definitie Nursing management of atrial septal defect
Nursing management of atrial septal defect Iatrogenic withdrawal syndrome
Iatrogenic withdrawal syndrome Iatrogenic
Iatrogenic Iatrogenic malnutrition
Iatrogenic malnutrition Iatrogenic
Iatrogenic Iatrogenic factors in periodontal disease
Iatrogenic factors in periodontal disease Iatrogenic fracture
Iatrogenic fracture Iatrogenic
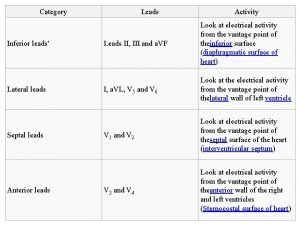
Iatrogenic Anterior leads
Anterior leads Conical sac
Conical sac Septal mi ekg
Septal mi ekg Idm antéro septal
Idm antéro septal Septal nuclei
Septal nuclei Papez circuit pathway
Papez circuit pathway Septal wall
Septal wall Lofotrik
Lofotrik Dr badi aldosari
Dr badi aldosari Diaphragm
Diaphragm Perforacion septal
Perforacion septal