Clean Room DATE PLACE Disclaimer This presentation contains
















- Slides: 26

Clean Room DATE PLACE Disclaimer: This presentation contains information on the general principles for safe handling of hazardous drugs. This presentation cannot account for individual variation among facilities and cannot be considered inclusive of all proper methods of occupational safety. It is the responsibility of the facility management and staff to determine the best course of occupational safety for operations. The American Cancer Society and its partners assume no responsibility for any injury or damage to persons or property arising out of or related to any use of these materials, or for any errors or omissions. Last updated: February 2020

Who should take this training Any staff person responsible for cleaning sterile drug compounding areas. These staff may include cleaners, technicians, nurses and pharmacists. 2

Training Modules + Learning Objectives Clean Room Overview • Describe a clean room. • Determine criteria for a clean room with a containment primary engineering control (CPEC). • List minimum personal protective equipment (PPE) requirements for cleaning the clean room Cleaning the Clean Room • List procedure for cleaning floors, walls, ceilings, work surfaces, carts, tables, and storage areas. • Identify materials not suitable for clean room use • List waste handling steps. Ante-Room • List additional criteria for cleaning the ante-room. 3

Clean Room Overview

Room definitions • Clean room: the area designated for preparing sterile products (i. e. , a room in which the concentration of airborne particles is controlled, that is constructed and used in a manner to minimize the introduction, generation, and retention of particles inside the room. The area should include: • Buffer zone/room: area in which the cleanest work surface (i. e. , ventilation tool) is located • Ante area/room: clean area that precedes the buffer zone/room, for donning of personal protective equipment (PPE) Reference 2 5

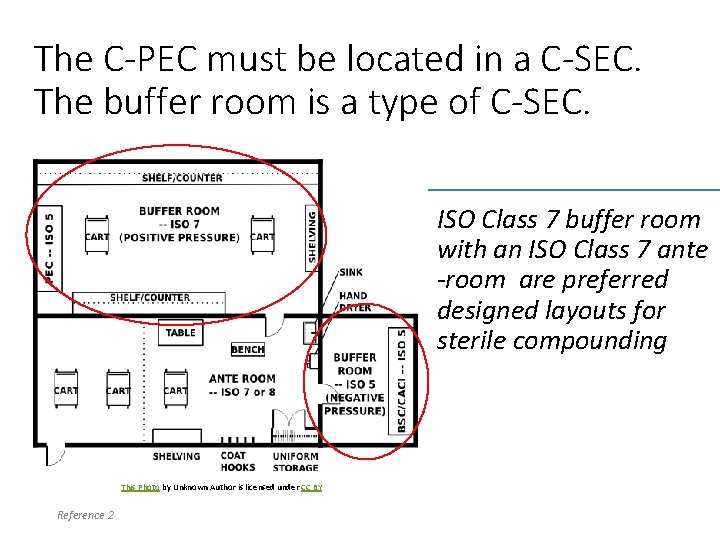
This Photo by Unknown Author is licensed under CC BY-NC-ND This Photo by Unknown Author is licensed under CC BY-SA A clean room is a controlled area. Clean rooms used for sterile drug compounding are a containment secondary engineering control (C-SEC ) or containment segregated compounding area (C-SCA) Reference 1 6

The C-PEC must be located in a C-SEC. The buffer room is a type of C-SEC. ISO Class 7 buffer room with an ISO Class 7 ante -room are preferred designed layouts for sterile compounding 7 This Photo by Unknown Author is licensed under CC BY Reference 2

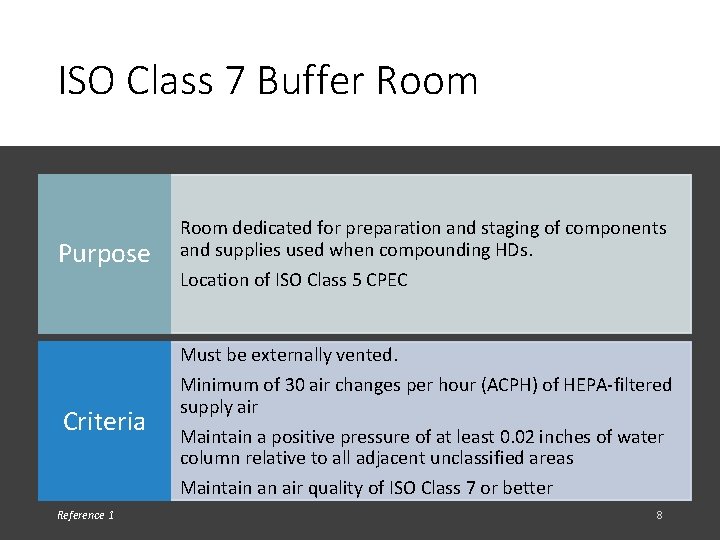
ISO Class 7 Buffer Room Purpose Room dedicated for preparation and staging of components and supplies used when compounding HDs. Location of ISO Class 5 CPEC Must be externally vented. Criteria Reference 1 Minimum of 30 air changes per hour (ACPH) of HEPA-filtered supply air Maintain a positive pressure of at least 0. 02 inches of water column relative to all adjacent unclassified areas Maintain an air quality of ISO Class 7 or better 8

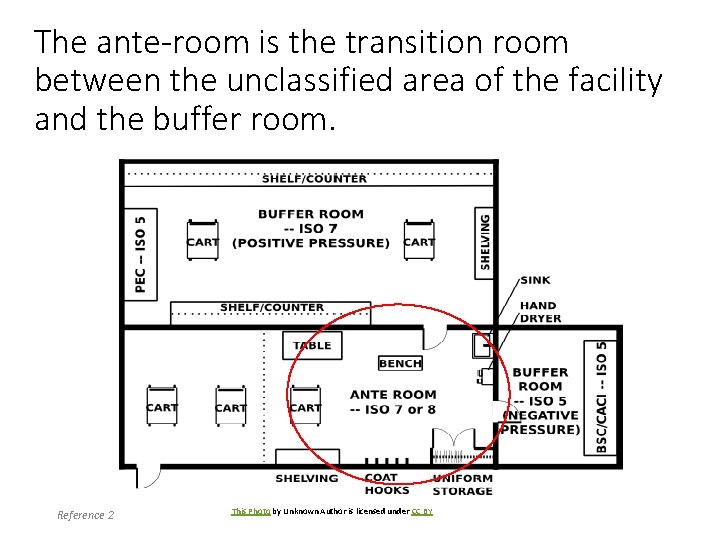
The ante-room is the transition room between the unclassified area of the facility and the buffer room. 9 Reference 2 This Photo by Unknown Author is licensed under CC BY

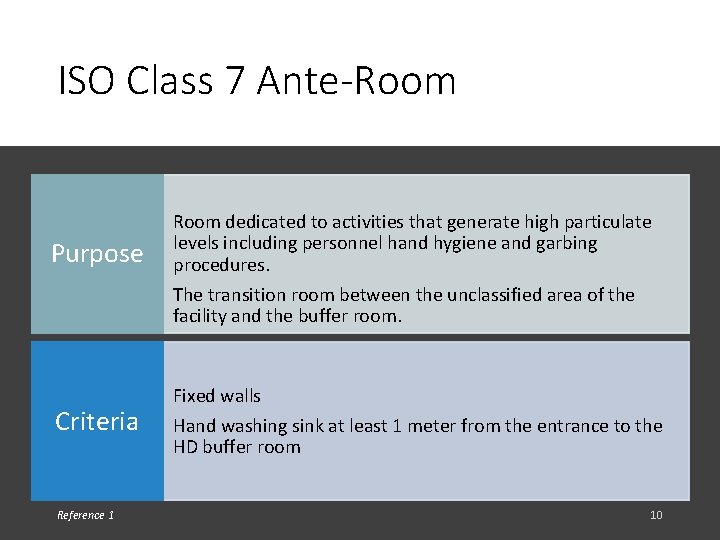
ISO Class 7 Ante-Room Purpose Criteria Reference 1 Room dedicated to activities that generate high particulate levels including personnel hand hygiene and garbing procedures. The transition room between the unclassified area of the facility and the buffer room. Fixed walls Hand washing sink at least 1 meter from the entrance to the HD buffer room 10

PPE Protective Gloves An N 95 respirator Disposable Gown Goggles Wear appropriate PPE for cleaning and decontaminating work. Reference 2 11

Cleaning the Clean Room

Buffer Room Example 13

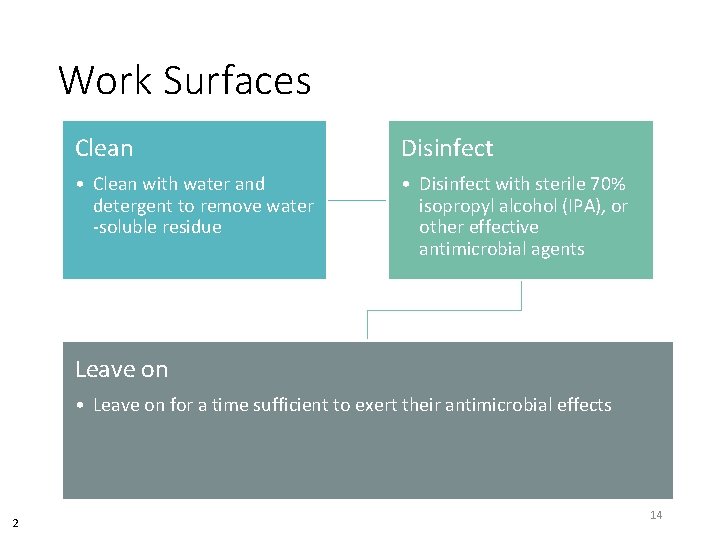
Work Surfaces Clean Disinfect • Clean with water and detergent to remove water -soluble residue • Disinfect with sterile 70% isopropyl alcohol (IPA), or other effective antimicrobial agents Leave on • Leave on for a time sufficient to exert their antimicrobial effects 2 14

Carts + Tables Carts and tables • Should be made of a material that can be easily cleaned and disinfected. Stainless steel is recommended Stools and chairs • Should be cleanroom quality Carts, tables, and stools • Should be cleaned and disinfected weekly and after any unanticipated event that could increase the risk of microbial contamination Reference 2 This Photo by Unknown Author is licensed under CC BY-SA 15

Storage Shelving This Photo by Unknown Author is licensed under CC BY Storage shelving is emptied of all supplies and then cleaned and disinfected at least weekly Reference 2

Not Suitable for Cleanroom Use Reference 2 Carpet Porous Floors Porous Walls Porous Ceiling This Photo by Unknown Author is licensed under CC BY 17

Floors in the cleanroom are cleaned by mopping at least once daily when no aseptic operations are in progress. Reference 2 This Photo by Unknown Author is licensed under CC BY-ND 18

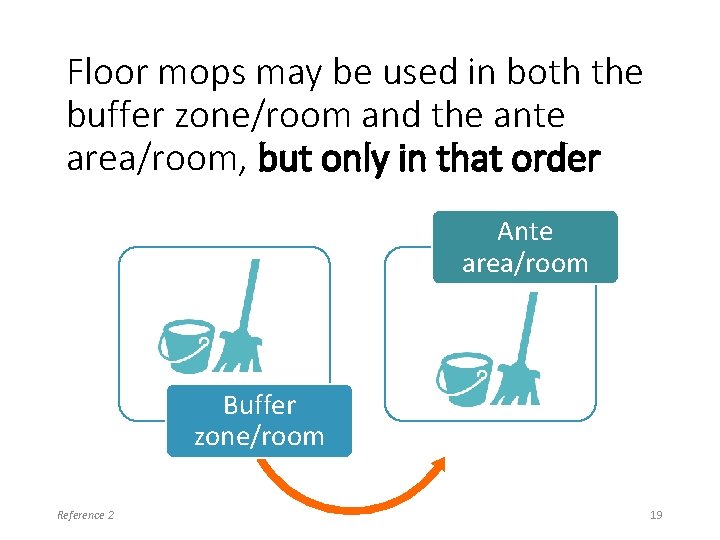
Floor mops may be used in both the buffer zone/room and the ante area/room, but only in that order Ante area/room Buffer zone/room Reference 2 19

Keep Storage Clean Refrigerators, freezers, shelves, and other areas where pharmacy-prepared sterile products are stored, should be kept clean. Reference 2 20

Other Equipment Reference 2 Equipment that does not come in contact with the finished product should be properly cleaned, rinsed, and disinfected before being placed in the clean room. 21

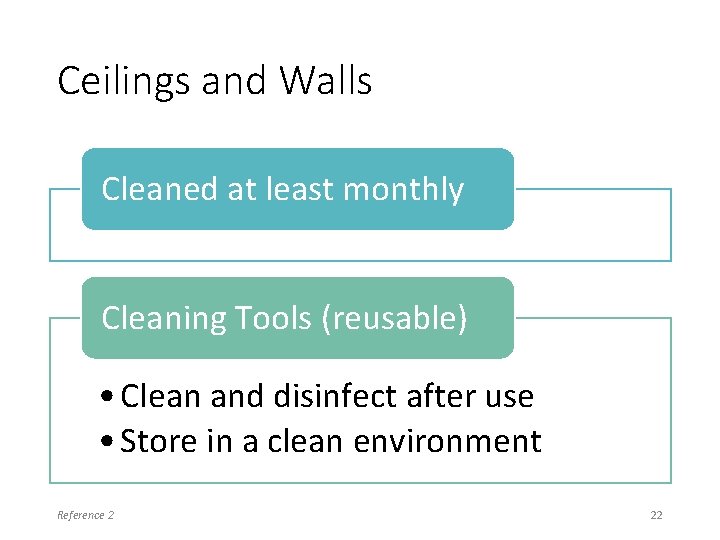
Ceilings and Walls Cleaned at least monthly Cleaning Tools (reusable) • Clean and disinfect after use • Store in a clean environment Reference 2 22

Decontamination of exterior of ventilation tool See “ Cleaning and Decontamination of Ventilation Tool” Training Reference 2 This Photo by Unknown Author is licensed under CC BY-SA-NC 23

Waste Handling An appropriate method of disposing of waste, including needles, should be established which does not allow accumulation in the cleanroom. Reference 2 24

In the Ante Room: Supplies and equipment If supplies are received in sealed pouches Should be wiped with a disinfectant after removal from shipping cartons The pouches can be removed as the supplies are introduced into the cleanroom without the need to disinfect the individual supply items No shipping or other external cartons may be taken into the cleanroom Reference 2 25

26 References 1. US Pharmacopeia. USP General Chapter <800> Hazardous Drugs-Handling in Healthcare Settings [Internet]. 2017 2. Connor T, Mc. Lauchlan R, Vandenbroucke J. ISOPP Standards of Practice: Safe Handling of Cytotoxics. J Oncol Pharm Pract. 2007; 13(1)