Chemistry I Chapters 15 16 Chemistry I HD

























- Slides: 51

Chemistry I – Chapters 15 & 16 Chemistry I HD – Chapter 15 ICP – Chapter 22 Solutions 1 SAVE PAPER AND INK!!! When you print out the notes on Power. Point, print "Handouts" instead of "Slides" in the print setup. Also, turn off the backgrounds (Tools>Options>Print>UNcheck "Background Printing")! Why does a raw egg swell or shrink when placed in different solutions?

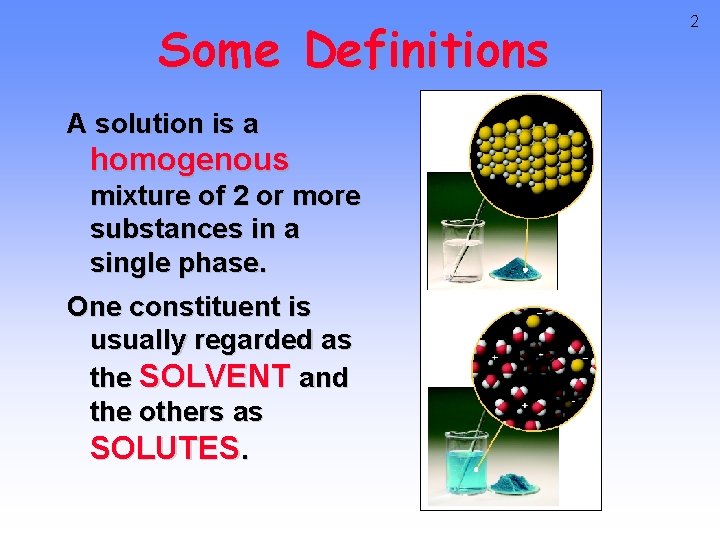
Some Definitions A solution is a homogenous mixture of 2 or more substances in a single phase. One constituent is usually regarded as the SOLVENT and the others as SOLUTES. 2

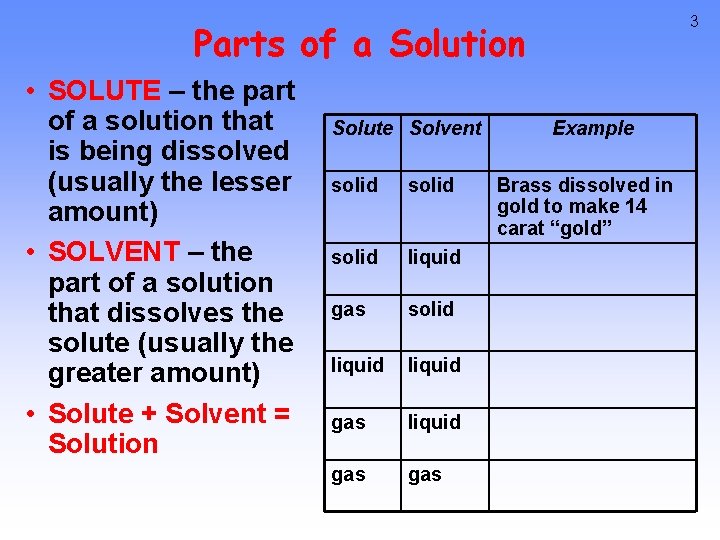
3 Parts of a Solution • SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) • SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) • Solute + Solvent = Solution Solute Solvent solid liquid gas solid liquid gas Example Brass dissolved in gold to make 14 carat “gold”

4 Aqueous solutions • When the solvent is water… » the solution is aqueous! • Remember (aq) from balancing chemical equations? – Ex. Na. Cl (s) + H 2 O => Na+ (aq) + Cl- (aq)

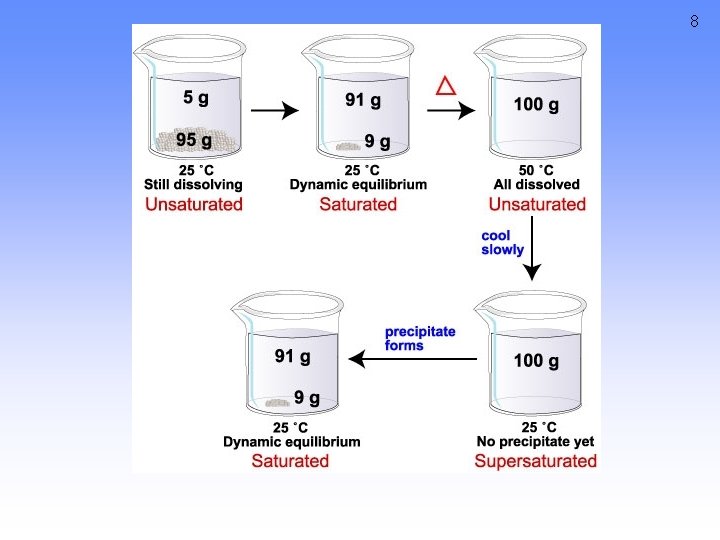
5 Classifying solutions A saturated solution contains the maximum quantity of solute that dissolves at that temperature. An unsaturated solution contains less than the maximum amount of solute that can dissolve at a particular temperature A supersaturated solution contains more dissolved solid than a saturated solution will hold at that temperature

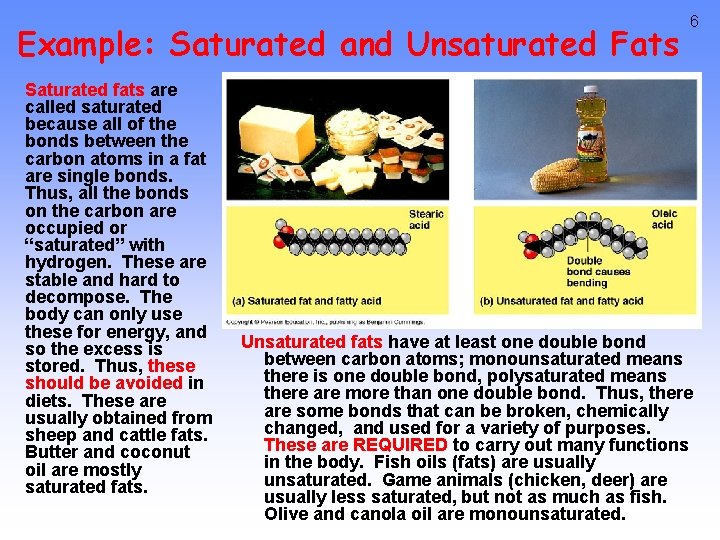
Example: Saturated and Unsaturated Fats Saturated fats are called saturated because all of the bonds between the carbon atoms in a fat are single bonds. Thus, all the bonds on the carbon are occupied or “saturated” with hydrogen. These are stable and hard to decompose. The body can only use these for energy, and so the excess is stored. Thus, these should be avoided in diets. These are usually obtained from sheep and cattle fats. Butter and coconut oil are mostly saturated fats. 6 Unsaturated fats have at least one double bond between carbon atoms; monounsaturated means there is one double bond, polysaturated means there are more than one double bond. Thus, there are some bonds that can be broken, chemically changed, and used for a variety of purposes. These are REQUIRED to carry out many functions in the body. Fish oils (fats) are usually unsaturated. Game animals (chicken, deer) are usually less saturated, but not as much as fish. Olive and canola oil are monounsaturated.

SUPERSATURATED SOLUTIONS *contain more solute than is possible to be dissolved Supersaturated solutions are unstable. The supersaturation is only temporary, and usually accomplished in one of two ways: 1. Warm the solvent so that it will dissolve more, then cool the solution 2. Evaporate some of the solvent carefully so that the solute does not solidify and come out of solution. 7

8

9 Supersaturated Sodium Acetate • One application of a supersaturated solution is the sodium acetate “heat pack. ” • Crystallization is triggered by flexing a disc of ferrous metal embedded in the liquid. Pressing the disc releases very tiny adhered crystals of sodium acetate into the solution which then act as nucleation sites for the crystallization of the sodium acetate into the hydrated salt (sodium acetate trihydrate). Because the liquid is supersaturated, this makes the solution crystallize suddenly, thereby releasing the energy of the crystal lattice. The pad can be reused by placing it in boiling water for 10– 15 minutes, which redissolves the sodium acetate trihydrate in the contained water and recreates a supersaturated solution. Once the pad has returned to room temperature it can be triggered again.

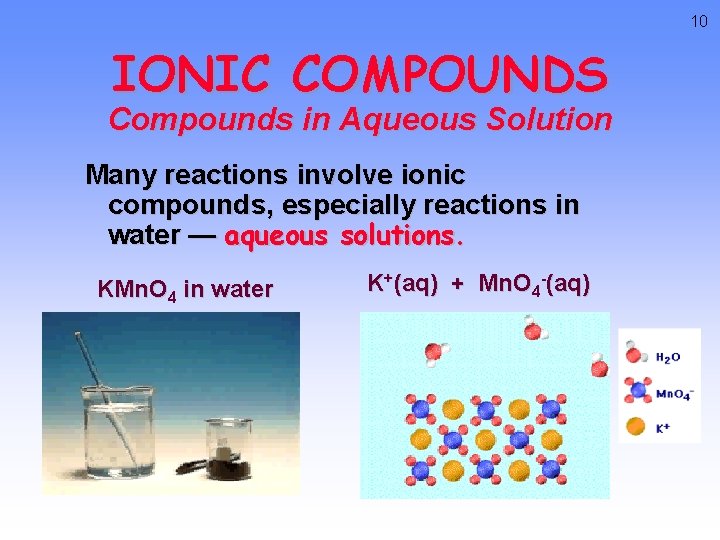
10 IONIC COMPOUNDS Compounds in Aqueous Solution Many reactions involve ionic compounds, especially reactions in water — aqueous solutions. KMn. O 4 in water K+(aq) + Mn. O 4 -(aq)

Aqueous Solutions How do we know ions are present in aqueous solutions? The solutions ________ They are called ELECTROLYTES HCl, Mg. Cl 2, and Na. Cl are strong electrolytes. They dissociate completely (or nearly so) into ions. 11

Aqueous Solutions Some compounds dissolve in water but do not conduct electricity. They are called nonelectrolytes. Examples include: sugar ethanol ethylene glycol 12

It’s Time to Play Everyone’s Favorite Game Show… Electrolyte or Nonelectrolyte! 13

14 Electrolytes in the Body Carry messages to and from the brain as electrical signals Maintain cellular function with the correct concentrations electrolytes Make your own 50 -70 g sugar One liter of warm water Pinch of salt 200 ml of sugar free fruit juice Mix, cool and drink

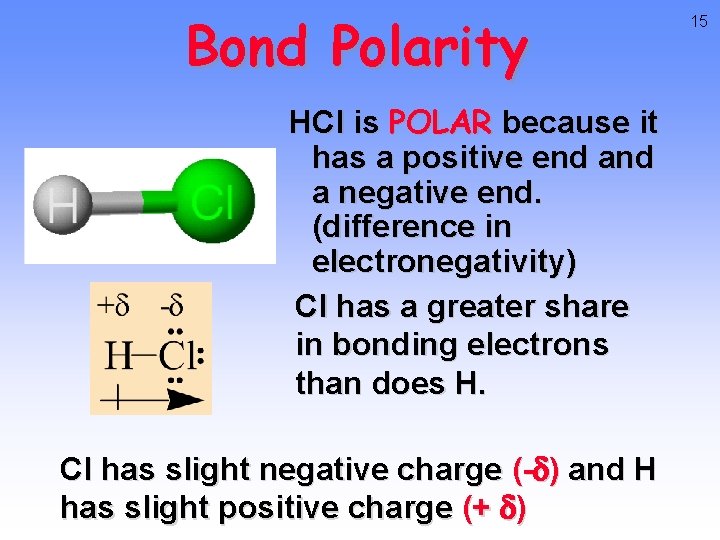
Bond Polarity HCl is POLAR because it has a positive end a negative end. (difference in electronegativity) Cl has a greater share in bonding electrons than does H. Cl has slight negative charge (-d) and H has slight positive charge (+ d) 15

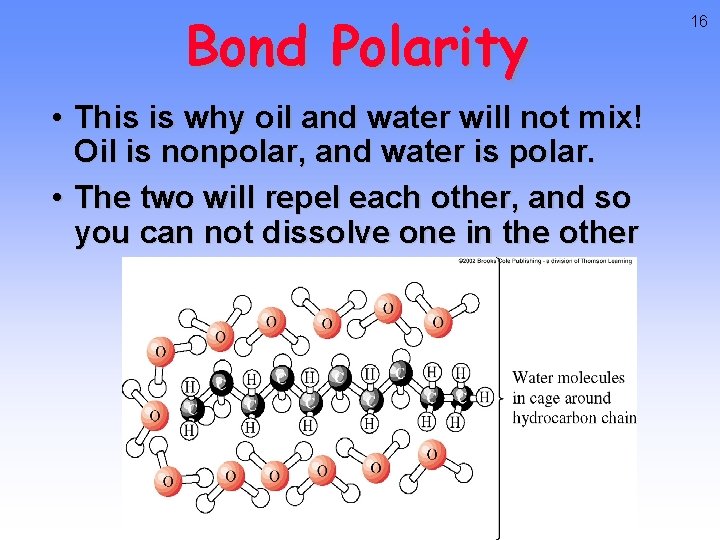
Bond Polarity • This is why oil and water will not mix! Oil is nonpolar, and water is polar. • The two will repel each other, and so you can not dissolve one in the other 16

Bond Polarity 17 • “Like Dissolves Like” –Polar dissolves Polar –Nonpolar dissolves Nonpolar

18 Aqueous solutions

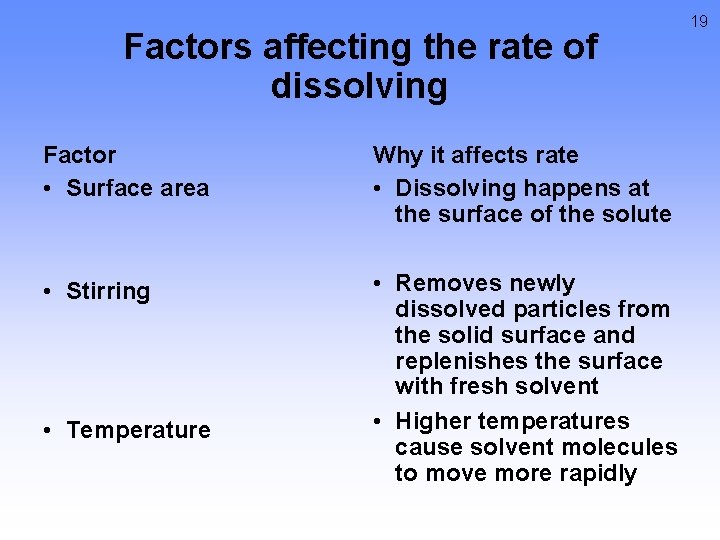
Factors affecting the rate of dissolving Factor • Surface area Why it affects rate • Dissolving happens at the surface of the solute • Stirring • Removes newly dissolved particles from the solid surface and replenishes the surface with fresh solvent • Higher temperatures cause solvent molecules to move more rapidly • Temperature 19

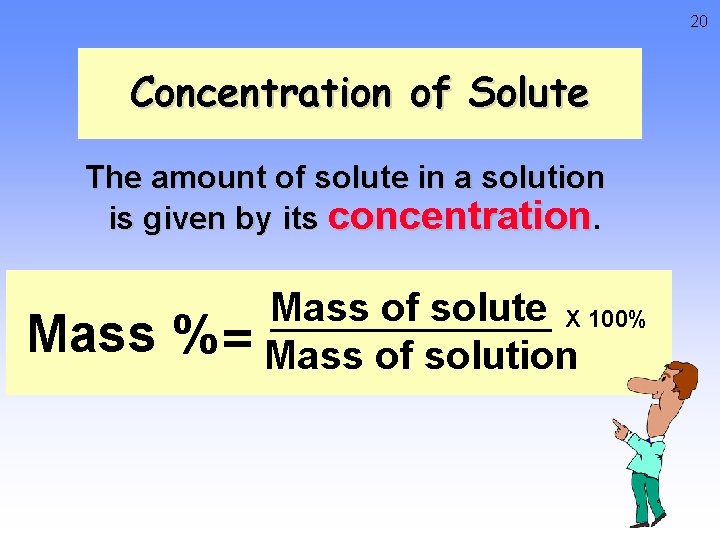
20 Concentration of Solute The amount of solute in a solution is given by its concentration. Mass of solute X 100% % = Mass of solution

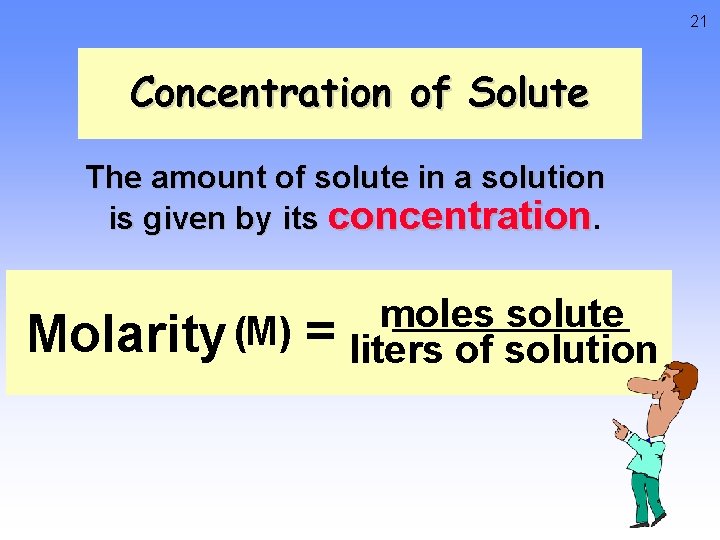
21 Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution

1. 0 L of water was used to make 1. 0 L of solution. Notice the water left over. 22

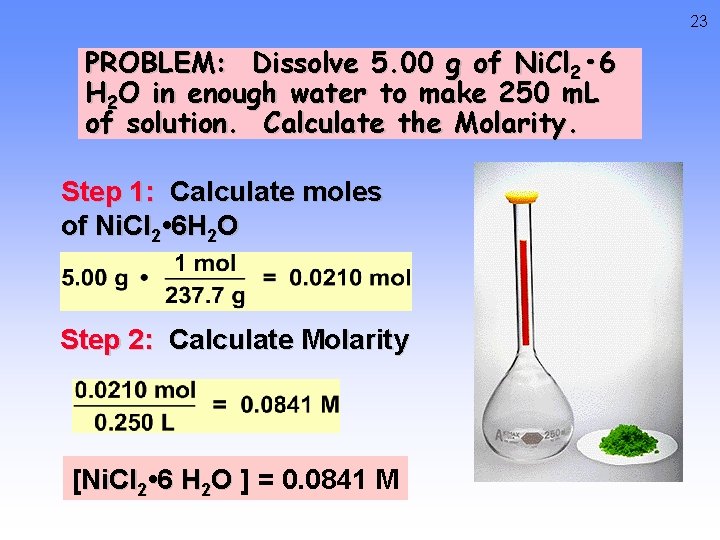
23 PROBLEM: Dissolve 5. 00 g of Ni. Cl 2 • 6 H 2 O in enough water to make 250 m. L of solution. Calculate the Molarity. Step 1: Calculate moles of Ni. Cl 2 • 6 H 2 O Step 2: Calculate Molarity [Ni. Cl 2 • 6 H 2 O ] = 0. 0841 M

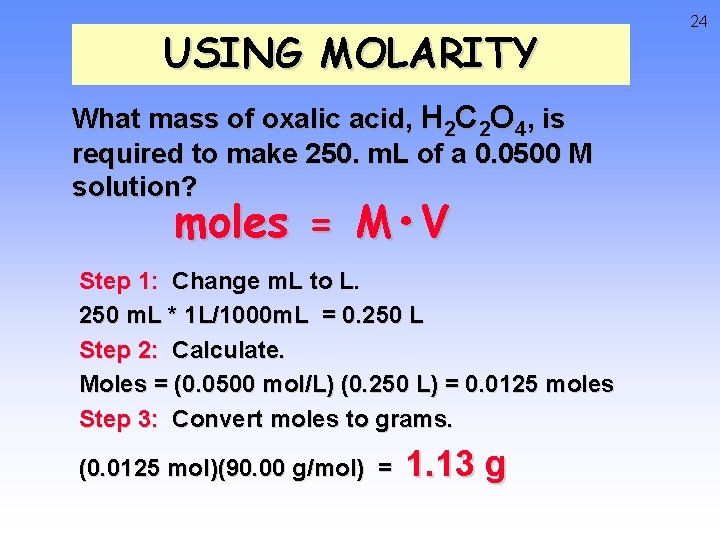
USING MOLARITY What mass of oxalic acid, H 2 C 2 O 4, is required to make 250. m. L of a 0. 0500 M solution? moles = M • V Step 1: Change m. L to L. 250 m. L * 1 L/1000 m. L = 0. 250 L Step 2: Calculate. Moles = (0. 0500 mol/L) (0. 250 L) = 0. 0125 moles Step 3: Convert moles to grams. (0. 0125 mol)(90. 00 g/mol) = 1. 13 g 24

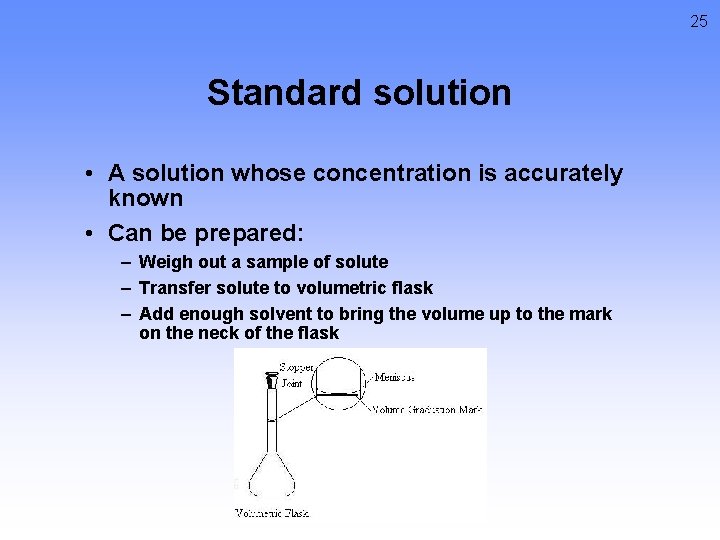
25 Standard solution • A solution whose concentration is accurately known • Can be prepared: – Weigh out a sample of solute – Transfer solute to volumetric flask – Add enough solvent to bring the volume up to the mark on the neck of the flask

26 Dilution • Chemists often keep standard solutions in stock and dilute them to the concentration they want • Dilution- the process of adding more solvent to a solution • Typical calculation: determining how much water must be added to an amount of stock solution to achieve a solution of the desired concentration – Only water is added – Amount of solute in final solution= amount of solute in original stock solution

27 Learning Check How many grams of Na. OH are required to prepare 400. m. L of 3. 0 M Na. OH solution? 1) 12 g 2) 48 g 3) 300 g

Concentration Units An IDEAL SOLUTION is one where the properties depend only on the concentration of solute. Need conc. units to tell us the number of solute particles per solvent particle. The unit “molarity” does not do this! 28

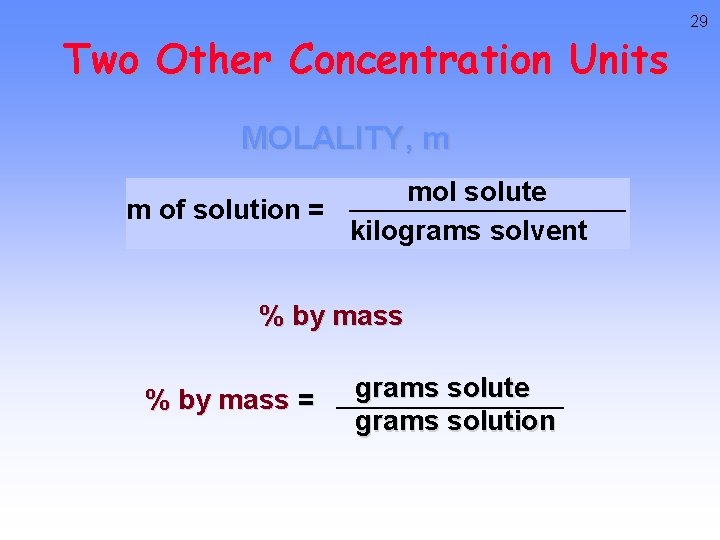
Two Other Concentration Units MOLALITY, m mol solute m of solution = kilograms solvent % by mass = grams solute grams solution 29

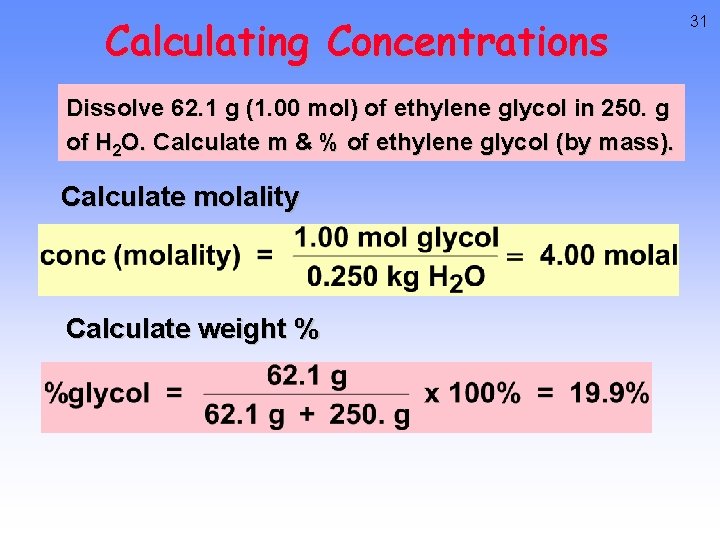
Calculating Concentrations Dissolve 62. 1 g (1. 00 mol) of ethylene glycol in 250. g of H 2 O. Calculate molality and % by mass of ethylene glycol. 30

Calculating Concentrations Dissolve 62. 1 g (1. 00 mol) of ethylene glycol in 250. g of H 2 O. Calculate m & % of ethylene glycol (by mass). Calculate molality Calculate weight % 31

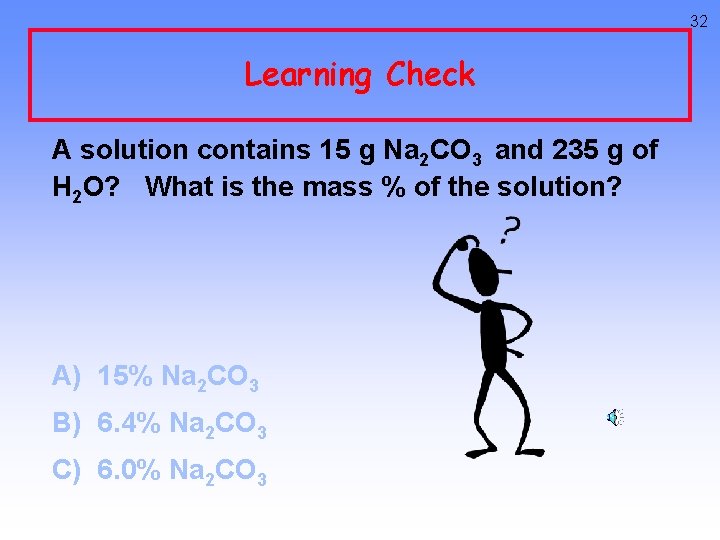
32 Learning Check A solution contains 15 g Na 2 CO 3 and 235 g of H 2 O? What is the mass % of the solution? A) 15% Na 2 CO 3 B) 6. 4% Na 2 CO 3 C) 6. 0% Na 2 CO 3

33 Using mass % How many grams of Na. Cl are needed to prepare 250 g of a 10. 0% (by mass) Na. Cl solution?

34 Try this molality problem • 25. 0 g of Na. Cl is dissolved in 5000. m. L of water. Find the molality (m) of the resulting solution. m = mol solute / kg solvent 25 g Na. Cl 1 mol Na. Cl 58. 5 g Na. Cl = 0. 427 mol Na. Cl Since the density of water is 1 g/m. L, 5000 m. L = 5000 g, which is 5 kg 0. 427 mol Na. Cl 5 kg water = 0. 0854 m salt water

Colligative Properties On adding a solute to a solvent, the properties of the solvent are modified. • Vapor pressure decreases • Melting point decreases • Boiling point increases • Osmosis is possible (osmotic pressure) These changes are called COLLIGATIVE PROPERTIES. They depend only on the NUMBER of solute particles relative to solvent particles, not on the KIND of solute particles. 35

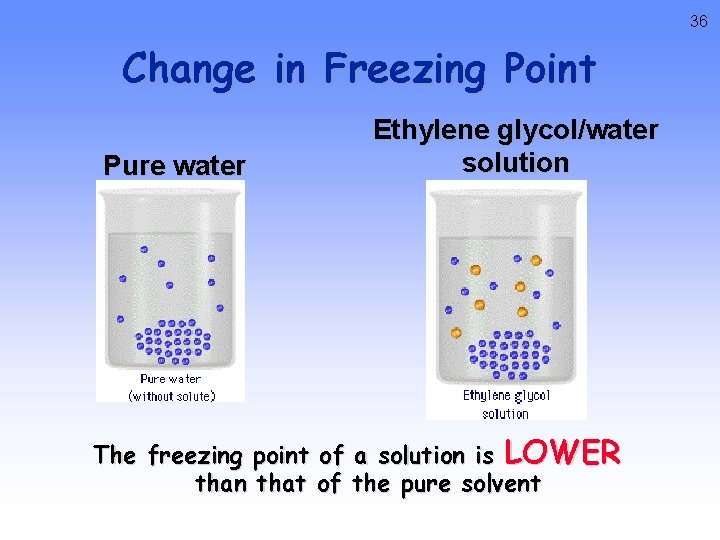
36 Change in Freezing Point Pure water Ethylene glycol/water solution The freezing point of a solution is LOWER than that of the pure solvent

Change in Freezing Point Common Applications of Freezing Point Depression Propylene glycol Ethylene glycol – deadly to small animals 37

Change in Freezing Point Common Applications of Freezing Point Depression Which would you use for the streets of Bloomington to lower the freezing point of ice and why? Would the temperature make any difference in your decision? a) sand, Si. O 2 b) Rock salt, Na. Cl c) Ice Melt, Ca. Cl 2 38

Change in Boiling Point Common Applications of Boiling Point Elevation 39

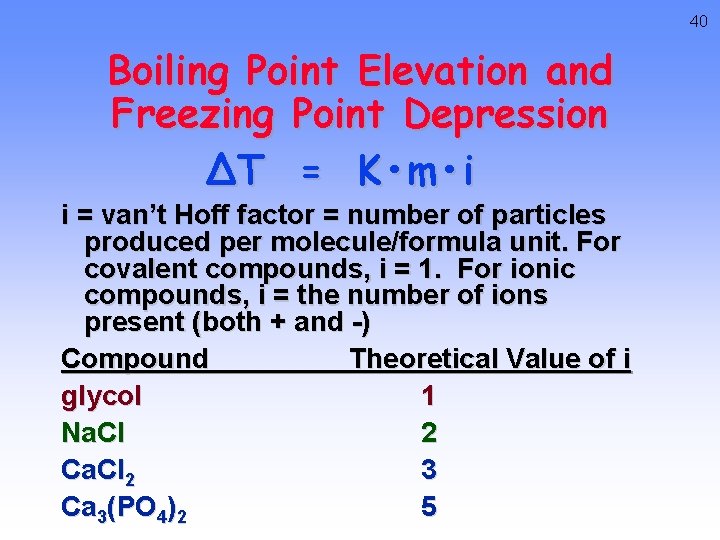
40 Boiling Point Elevation and Freezing Point Depression ∆T = K • m • i i = van’t Hoff factor = number of particles produced per molecule/formula unit. For covalent compounds, i = 1. For ionic compounds, i = the number of ions present (both + and -) Compound Theoretical Value of i glycol 1 Na. Cl 2 Ca. Cl 2 3 Ca 3(PO 4)2 5

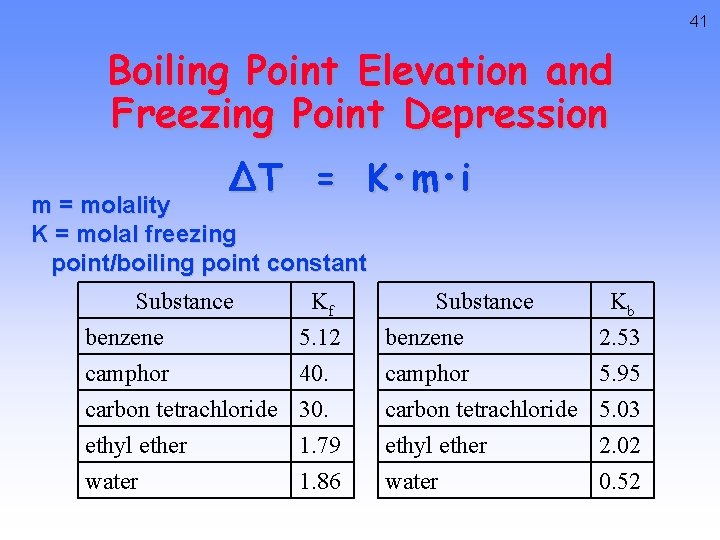
41 Boiling Point Elevation and Freezing Point Depression ∆T = K • m • i m = molality K = molal freezing point/boiling point constant Substance Kf benzene 5. 12 camphor 40. carbon tetrachloride 30. Substance Kb benzene 2. 53 camphor 5. 95 carbon tetrachloride 5. 03 ethyl ether water 1. 79 1. 86 2. 02 0. 52

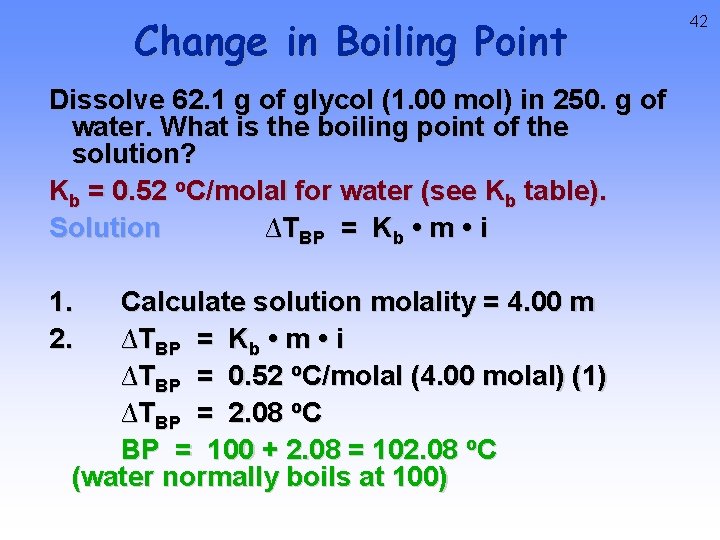
Change in Boiling Point Dissolve 62. 1 g of glycol (1. 00 mol) in 250. g of water. What is the boiling point of the solution? Kb = 0. 52 o. C/molal for water (see Kb table). Solution ∆TBP = Kb • m • i 1. 2. Calculate solution molality = 4. 00 m ∆TBP = Kb • m • i ∆TBP = 0. 52 o. C/molal (4. 00 molal) (1) ∆TBP = 2. 08 o. C BP = 100 + 2. 08 = 102. 08 o. C (water normally boils at 100) 42

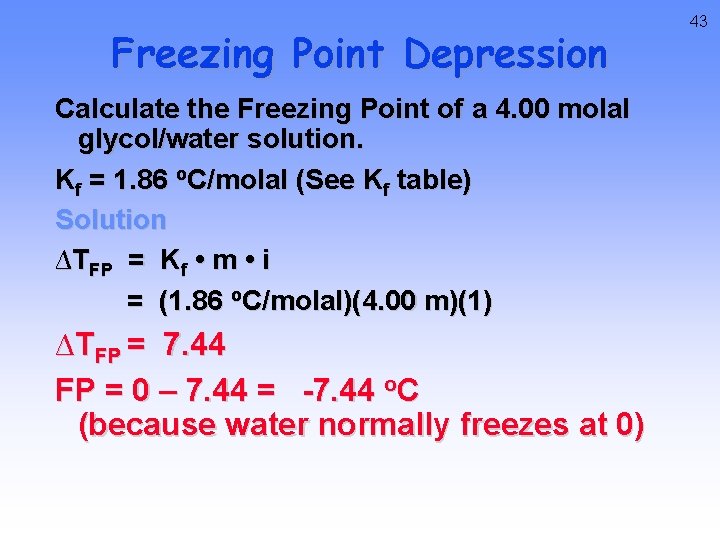
Freezing Point Depression Calculate the Freezing Point of a 4. 00 molal glycol/water solution. Kf = 1. 86 o. C/molal (See Kf table) Solution ∆TFP = Kf • m • i = (1. 86 o. C/molal)(4. 00 m)(1) ∆TFP = 7. 44 FP = 0 – 7. 44 = -7. 44 o. C (because water normally freezes at 0) 43

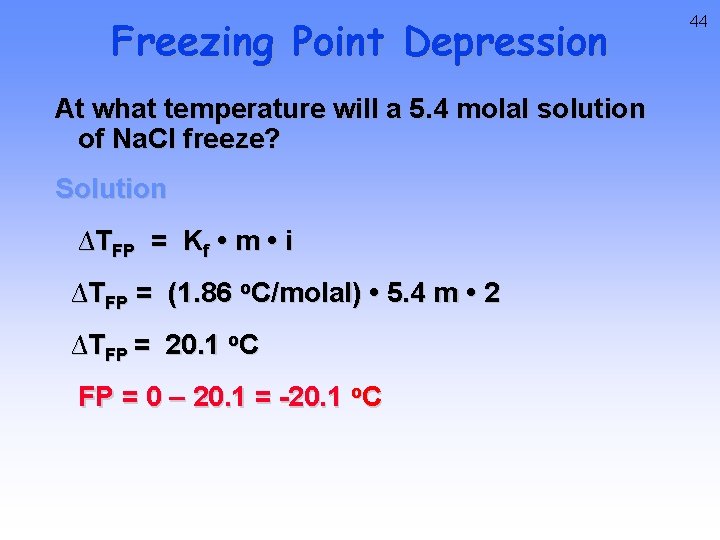
Freezing Point Depression At what temperature will a 5. 4 molal solution of Na. Cl freeze? Solution ∆TFP = Kf • m • i ∆TFP = (1. 86 o. C/molal) • 5. 4 m • 2 ∆TFP = 20. 1 o. C FP = 0 – 20. 1 = -20. 1 o. C 44

45 Preparing Solutions • Weigh out a solid solute and dissolve in a given quantity of solvent. • Dilute a concentrated solution to give one that is less concentrated.

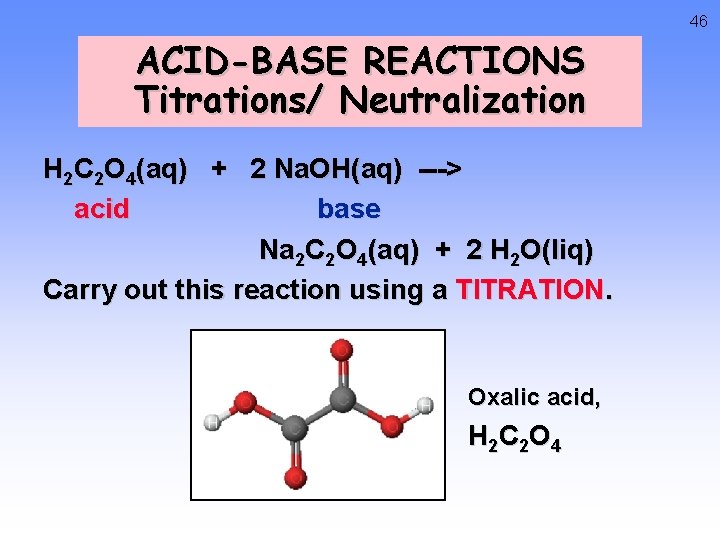
46 ACID-BASE REACTIONS Titrations/ Neutralization H 2 C 2 O 4(aq) + 2 Na. OH(aq) ---> acid base Na 2 C 2 O 4(aq) + 2 H 2 O(liq) Carry out this reaction using a TITRATION. Oxalic acid, H 2 C 2 O 4

Setup for titrating an acid with a base 47

48 Titration 1. Add solution from the buret. 2. Reagent (base) reacts with compound (acid) in solution in the flask. 3. Indicator shows when exact stoichiometric reaction has occurred. (Acid = Base) This is called NEUTRALIZATION.

49 Normality • Another unit of concentration we will use when discussing acids and bases • Focuses on H+ (from acid) and OH- (from base) ions • Equivalent of acid- amount of acid that can furnish 1 mol of H+ ions • Equivalent of base- amount of base that can furnish 1 mol of OH- ions • Equivalent weight- mass in grams of 1 equivalent of acid or base • Normality (N)= number of equivalents/ liter of solution

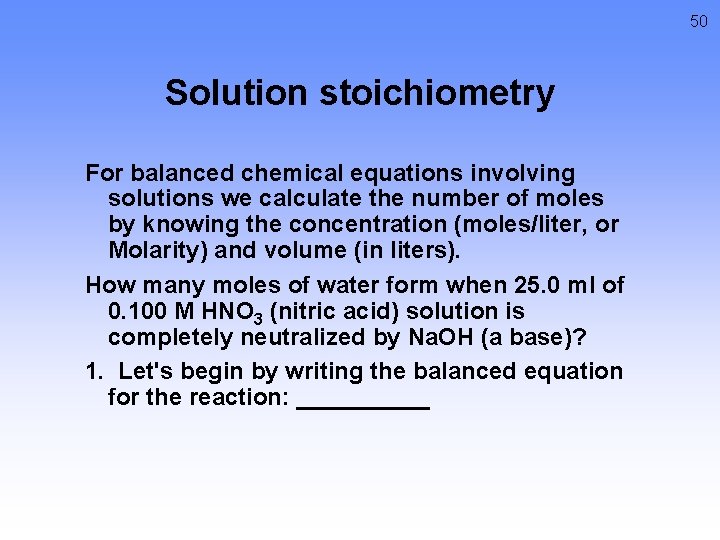
50 Solution stoichiometry For balanced chemical equations involving solutions we calculate the number of moles by knowing the concentration (moles/liter, or Molarity) and volume (in liters). How many moles of water form when 25. 0 ml of 0. 100 M HNO 3 (nitric acid) solution is completely neutralized by Na. OH (a base)? 1. Let's begin by writing the balanced equation for the reaction: _____

51 Stoichiometry ex. continued 2. The stoichiometric relationship between HNO 3 and H 2 O is __HNO 3: __H 2 O, therefore, for ___ mole of HNO 3 that is completely consumed (i. e. neutralized) in the reaction, ___ mole of H 2 O is produced. 3. How many moles of HNO 3 are we starting with? _____ 4. How many moles of water will this produce? _____
 Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Inorganic vs organic chemistry
Inorganic vs organic chemistry Ib chemistry organic chemistry
Ib chemistry organic chemistry Dracula chapter 2 summary
Dracula chapter 2 summary All deadlocks involve conflicting needs for
All deadlocks involve conflicting needs for Frankenstein chapter 11-16 summary
Frankenstein chapter 11-16 summary Great expectations chapter 9
Great expectations chapter 9 Pickwick hiding place
Pickwick hiding place To kill a mockingbird summary chapter 4
To kill a mockingbird summary chapter 4 Outliers book chapters
Outliers book chapters Catcher in the rye sunny
Catcher in the rye sunny The outsiders chapter 1-4 quiz
The outsiders chapter 1-4 quiz Frankenstein chapters 6-10 summary
Frankenstein chapters 6-10 summary Portia nelson autobiography in five short chapters
Portia nelson autobiography in five short chapters To kill a mockingbird chapter 7-8 summary
To kill a mockingbird chapter 7-8 summary To kill a mockingbird chapter 12-15 summary
To kill a mockingbird chapter 12-15 summary Things fall apart chapter 14-19 summary
Things fall apart chapter 14-19 summary Master naturalist san antonio
Master naturalist san antonio Chapter 1 brave new world
Chapter 1 brave new world Portia nelson autobiography in five short chapters
Portia nelson autobiography in five short chapters The wave chapters
The wave chapters Freak the mighty vocabulary chapters 1-4
Freak the mighty vocabulary chapters 1-4 Crescendo the giver
Crescendo the giver Nonviolent communication chapters
Nonviolent communication chapters Percy jackson vocabulary chapters 5-8
Percy jackson vocabulary chapters 5-8 Night chapter 1 and 2 quiz
Night chapter 1 and 2 quiz Active reading frankenstein chapters 11-16
Active reading frankenstein chapters 11-16 What happens in to kill a mockingbird chapter 12
What happens in to kill a mockingbird chapter 12 Chapter 1 you are the driver worksheet answers
Chapter 1 you are the driver worksheet answers Setting of things fall apart
Setting of things fall apart Genesis 6 9 22
Genesis 6 9 22 Reluctant fundamentalist chapter summary
Reluctant fundamentalist chapter summary To kill a mockingbird quiz
To kill a mockingbird quiz Chapter 11-12 scarlet letter
Chapter 11-12 scarlet letter Things fall apart chapters 4-6
Things fall apart chapters 4-6 Why did cole beat up peter
Why did cole beat up peter Summary of chapter 23 and 24 of the scarlet letter
Summary of chapter 23 and 24 of the scarlet letter Esperanza rising page count
Esperanza rising page count Growing up quotes in to kill a mockingbird chapter 1-5
Growing up quotes in to kill a mockingbird chapter 1-5 Summary of things fall apart chapter 20
Summary of things fall apart chapter 20 Jekyll and hyde chapter 3
Jekyll and hyde chapter 3 Deadlock summary by chapters
Deadlock summary by chapters Frankenstein chapters 11-16 summary
Frankenstein chapters 11-16 summary Unbroken chapter 1-5 summary
Unbroken chapter 1-5 summary Chapter 8 of great gatsby summary
Chapter 8 of great gatsby summary To kill a mockingbird chapter 28 summary
To kill a mockingbird chapter 28 summary Pda reading
Pda reading Catcher in the rye chapter 27
Catcher in the rye chapter 27 Charlie gotso
Charlie gotso Chapter 9 summary of lord of the flies
Chapter 9 summary of lord of the flies The village by the sea chapter 1 summary
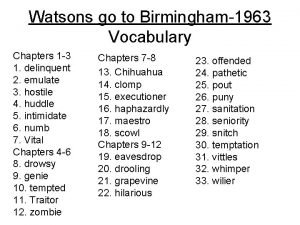
The village by the sea chapter 1 summary The watsons go to birmingham vocabulary chapters 1-3
The watsons go to birmingham vocabulary chapters 1-3