Chem 31 1018 Lecture Announcements Quiz 3 Today







- Slides: 18

Chem. 31 – 10/18 Lecture

Announcements • Quiz 3 Today • Today’s Lecture – Chapter 6 • Acids, Bases and Salts – Chapter 7 – Titrations • Overview and Definitions

Acids, Bases and Salts Definitions of Acids and Bases - Lewis Acids/Bases (defined before, most general category) - Brønsted-Lowry Acids/Bases: acid = proton donor base = proton acceptor (must have free electron pair so also is a Lewis base) - definitions are relative

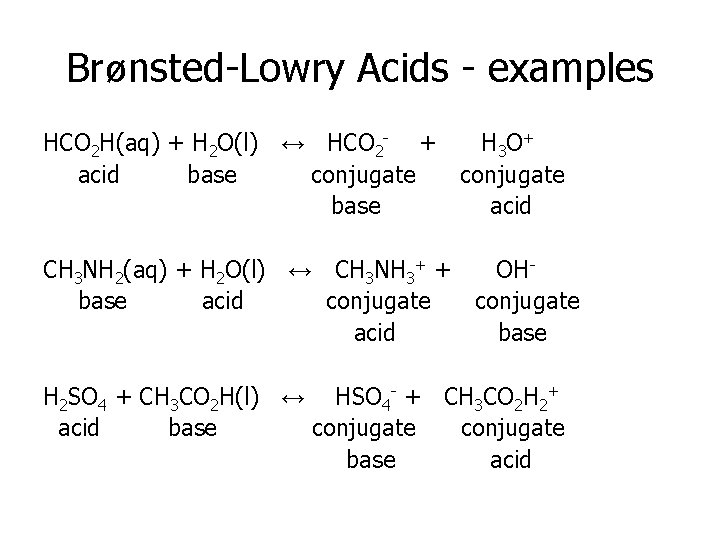
Brønsted-Lowry Acids - examples HCO 2 H(aq) + H 2 O(l) ↔ HCO 2 - + H 3 O+ acid base conjugate base acid CH 3 NH 2(aq) + H 2 O(l) ↔ CH 3 NH 3+ + base acid conjugate acid OHconjugate base H 2 SO 4 + CH 3 CO 2 H(l) ↔ HSO 4 - + CH 3 CO 2 H 2+ acid base conjugate base acid

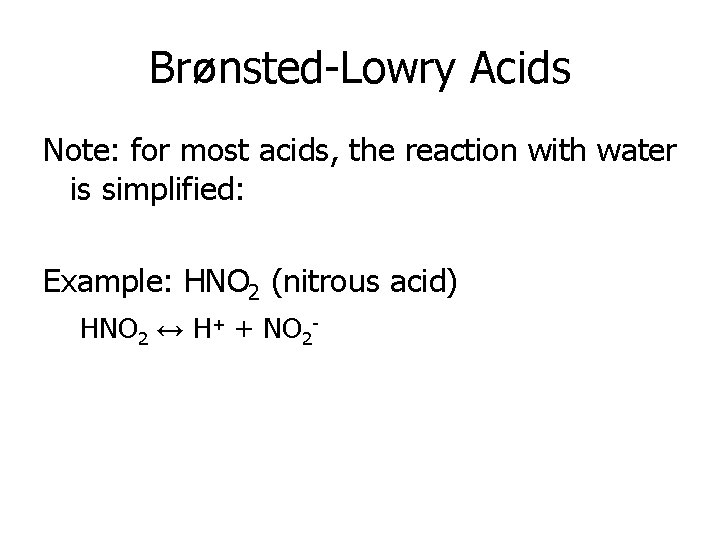
Brønsted-Lowry Acids Note: for most acids, the reaction with water is simplified: Example: HNO 2 (nitrous acid) HNO 2 ↔ H+ + NO 2 -

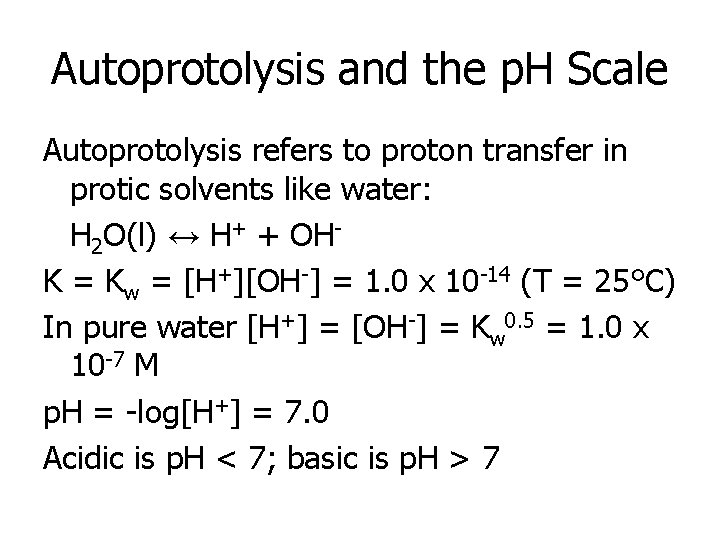
Autoprotolysis and the p. H Scale Autoprotolysis refers to proton transfer in protic solvents like water: H 2 O(l) ↔ H+ + OHK = Kw = [H+][OH-] = 1. 0 x 10 -14 (T = 25°C) In pure water [H+] = [OH-] = Kw 0. 5 = 1. 0 x 10 -7 M p. H = -log[H+] = 7. 0 Acidic is p. H < 7; basic is p. H > 7

Strong Acids • Strong acids completely dissociate in water (except at very high concentrations) – HX(aq) → H+ + X- (no HX(aq) exists) • Ka > 1 • Major strong acids: HCl, HNO 3, H 2 SO 4 • Note: – For H 2 SO 4, 1 st dissociation is that of a strong acid, but 2 nd dissociation is that of a weak acid (Ka ~ 0. 01)

Weak Acids • • Partially dissociate in water Most have H that can dissociate HX(aq) ↔ H+ + X- (HX(aq) exists) Example: HNO 2 ↔ H+ + NO 2 Degree of dissociation given by Ka value Ka = [H+][NO 2 -]/[HNO 2] Metal cations can be acids through the reaction: Mn+ + H 2 O(l) ↔ MOH(n-1)+ + H+ (although for +1 and some +2 metals the above reactions favor reactants so strongly the metals can be considered “neutral”)

Ionic Compounds in Water • First step should be dissociation to respective ions: example: Na. Cl(s) → Na+ + Cl • In subsequent steps, determine how anion/cation react: - anions usually only react as bases - cations may react as acids - see if ions are recognizable conjugate acids or bases - polyprotic acids are somewhat different

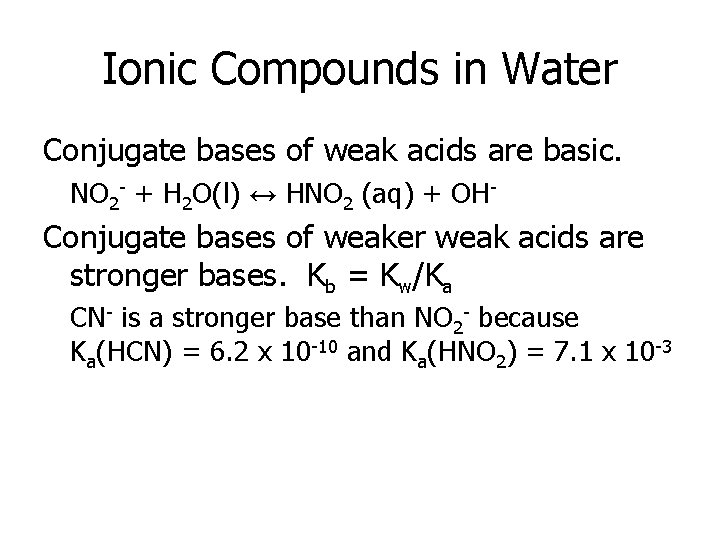
Ionic Compounds in Water Conjugate bases of weak acids are basic. NO 2 - + H 2 O(l) ↔ HNO 2 (aq) + OH- Conjugate bases of weaker weak acids are stronger bases. Kb = Kw/Ka CN- is a stronger base than NO 2 - because Ka(HCN) = 6. 2 x 10 -10 and Ka(HNO 2) = 7. 1 x 10 -3

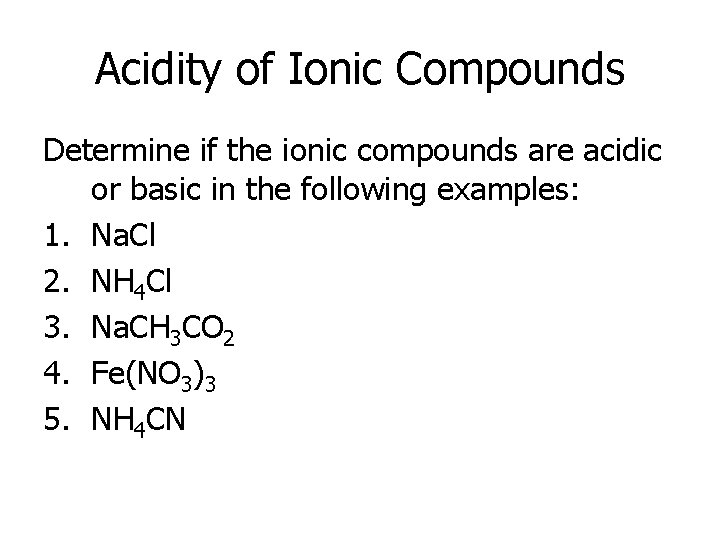
Acidity of Ionic Compounds Determine if the ionic compounds are acidic or basic in the following examples: 1. Na. Cl 2. NH 4 Cl 3. Na. CH 3 CO 2 4. Fe(NO 3)3 5. NH 4 CN

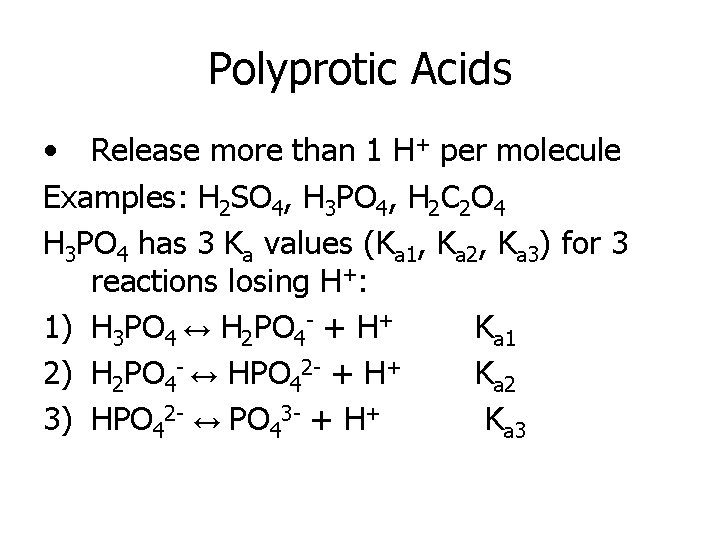
Polyprotic Acids • Release more than 1 H+ per molecule Examples: H 2 SO 4, H 3 PO 4, H 2 C 2 O 4 H 3 PO 4 has 3 Ka values (Ka 1, Ka 2, Ka 3) for 3 reactions losing H+: 1) H 3 PO 4 ↔ H 2 PO 4 - + H+ Ka 1 2) H 2 PO 4 - ↔ HPO 42 - + H+ Ka 2 3) HPO 42 - ↔ PO 43 - + H+ Ka 3

Chapter 7 - Titrations Introduction – Overview A. Chapter 7 covers general titrations (quantitation, practical aspects, types of titrations, shape of precipitation titration curve – not covering calculations due to time) B. Chapter 11 covers titration curves for acidbase titrations - covered later C. Other Chapters (12, 16) cover other types of titrations – not covered

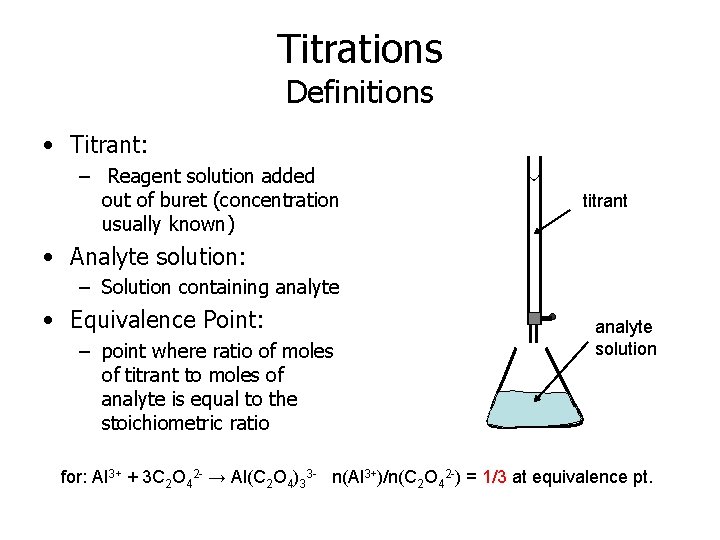
Titrations Definitions • Titrant: – Reagent solution added out of buret (concentration usually known) titrant • Analyte solution: – Solution containing analyte • Equivalence Point: – point where ratio of moles of titrant to moles of analyte is equal to the stoichiometric ratio analyte solution for: Al 3+ + 3 C 2 O 42 - → Al(C 2 O 4)33 - n(Al 3+)/n(C 2 O 42 -) = 1/3 at equivalence pt.

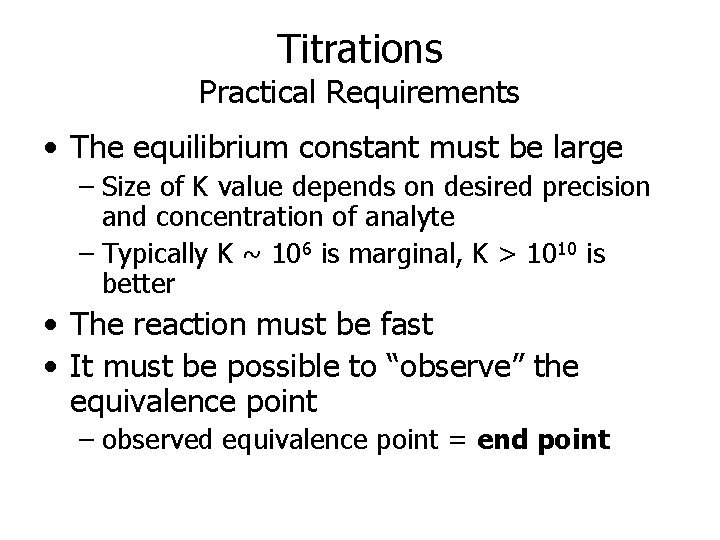
Titrations Practical Requirements • The equilibrium constant must be large – Size of K value depends on desired precision and concentration of analyte – Typically K ~ 106 is marginal, K > 1010 is better • The reaction must be fast • It must be possible to “observe” the equivalence point – observed equivalence point = end point

Titrations Detection of Endpoints • An endpoint is defined as the point in the titration when the equivalence point is observed • Ways to detect endpoints: – Use of colored reactants example: Mn. O 4 - + H 2 C 2 O 4 (aq) → Mn 2+ + CO 2 (g) PINK Clear – Use of indicators An indicator changes color in response to the change in a reactant’s concentration – Use of simple instruments Must respond quickly, but typical equipment is low cost

Titrations Detection of Endpoints • Simple instruments – electrodes (typically respond to log of ion concentrations) – spectroscopic measurements (measurement of absorption of light) – Can improve titration precision vs. using indicators • Titration Error = Difference between end point and equivalence point = systematic error • Note: It is possible to have large errors or uncertainties in detection of reagent conc. by various methods without having great titration errors to meter

Titrations Other Definitions • Standardization vs. Analyte Titrations – To accurately determine an analyte’s concentration, the titrant concentration must be well known – This can be done by preparing a primary standard (high purity standard) – Alternatively, the titrant concentration can be determined in a standardization titration (e. g. vs. a known standard) • Rationale: – many solutions can not be prepared accurately from available standards • Example: – determination of [H 2 O 2] by titration with Mn. O 4– neither compound is very stable so no primary standard – instead, [Mn. O 4 -] determined by titration with H 2 C 2 O 4 in standardization titration – then, H 2 O 2 titrated using standardized Mn. O 4 -
 Chemsheets
Chemsheets Erika p björkdahl
Erika p björkdahl Chem quiz.net
Chem quiz.net Pvu market cap
Pvu market cap /r/announcements
/r/announcements David ritthaler
David ritthaler What did mildred regret losing in the fire
What did mildred regret losing in the fire Kluver bucy syndrome
Kluver bucy syndrome General announcements
General announcements 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad For today's meeting
For today's meeting There is class today
There is class today Proposal kickoff meeting agenda
Proposal kickoff meeting agenda Characteristic of fingerprint
Characteristic of fingerprint Today's lesson or today lesson
Today's lesson or today lesson Example of repitition
Example of repitition No quiz today
No quiz today No quiz today
No quiz today Today's one question quiz
Today's one question quiz