Chem 11 ch 15 wkst 1 solutions 1




















- Slides: 22

Chem 11 ch 15 wkst 1 solutions

1. Define solution. A homogeneous mixture of two or more substances in a single physical state.

2. State 3 properties of solutions as discussed on page 502. Particles are very small in a solution. Particles are evenly distributed. Particles will not separate.

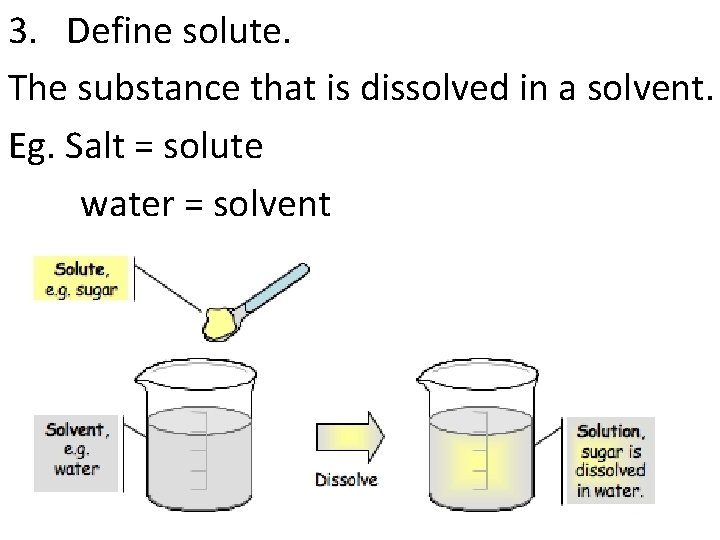
3. Define solute. The substance that is dissolved in a solvent. Eg. Salt = solute water = solvent

4. Define solvent. The substance that does the dissolving. Eg. Salt = solute water = solvent

5. Define soluble. The ability of a substance to dissolve in another substance. Usually means dissolves.

6. Define insoluble. The inability of a substance to dissolve in another substance. ie does not dissolve.

7. What are the 3 types of solutions and give and example of each. Solid solution Sterling silver = copper and silver Liquid solution Alcohol / water, salt/water, O 2 / water Gas solution Air = oxygen, nitrogen, argon, CO 2



8. Define miscible. The ability of a liquid to form a solution with another liquid in all proportions. Eg. The liquids will mix with each other like alcohol and water.

9. Define immiscible. The inability of a liquid to form a solution with another liquid in all proportions. Eg. The liquids will NOT mix with each other like oil and water

10. Define aqueous solutions. A solution in which the solvent is water

11. Define electrolyte. A substance that dissolves in water to form a solution that will conduct electricity. Eg. Salt dissolved in water = electrolytic solution due to the sodium ions and chloride ions.

12. What is the difference between an electrolyte solution and a nonelectrolyte solution and give an example of each. Electrolyte solution conducts electricity (like salt dissolved in water). Non-electrolytic solution does not conduct electricity ( like sugar dissolved in water)

13. Define concentration of solution. The amount of solute dissolved in a given amount of solution / solvent.

14. Define molarity and write the equation. The concentration of a solution in moles per liter. M = moles liter
15. What is a polar solvent? Give some examples. A liquid made up of polar molecules. Eg. Water, alcohol, acetone, acetic acid ( vinegar.

16. What is a non-polar solvent? Give some examples. A solution that is made up of non-polar molecules. Eg. Gasoline ( hexane ) oils, wax, grease, carbon tetrachloride

17. What is the rule concerning the dissolving of solutes in solvents? See chart page 515. Likes dissolves LIKE !! Polar molecules will dissolve in polar solvents. Non-polar molecules dissolve in non-polar solvents.


 Science olympiad san diego
Science olympiad san diego Chem
Chem Chem 301 gas law simulator
Chem 301 gas law simulator Dyno chem
Dyno chem Chem libretext
Chem libretext Chem 109
Chem 109 Chem pharma impex
Chem pharma impex Chem
Chem Chem
Chem Principles of kmt
Principles of kmt Chem
Chem Ap chemistry big idea 6 review answers
Ap chemistry big idea 6 review answers Energy quanta
Energy quanta Chem
Chem Chem 253
Chem 253 Chem moles
Chem moles Factors affecting vapor pressure
Factors affecting vapor pressure Pub chem
Pub chem Ap chem equilibrium
Ap chem equilibrium Ario acronym chemistry
Ario acronym chemistry Dipole induced dipole interaction
Dipole induced dipole interaction Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Octahedral bond angle
Octahedral bond angle