Chapter 8 What Is Matter Essential Questions I





















- Slides: 32

Chapter 8 What Is Matter?

Essential Questions I can identify the parts of an atom and its purpose I can explain why mixtures can be separated using physical properties. I can classify elements and compounds according to their properties. I can compare properties of different combinations of elements. ,

8. 1 Matter and Atoms Matter Anything that takes up space and has mass. What is Matter?

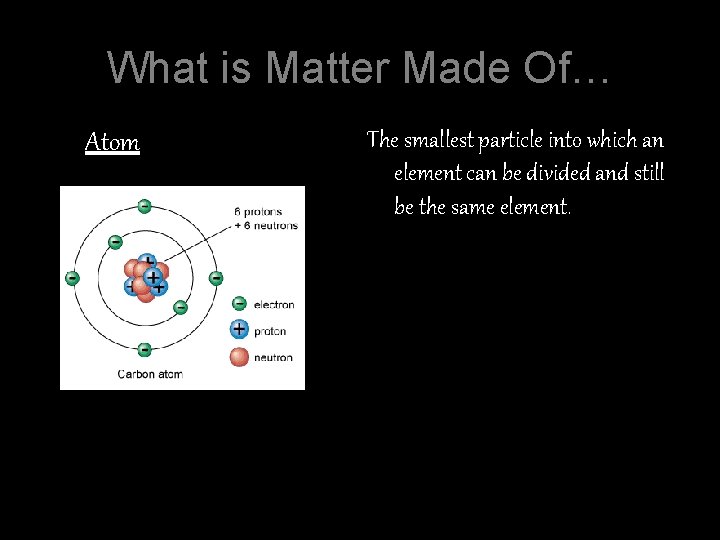
What is Matter Made Of… Atom The smallest particle into which an element can be divided and still be the same element.

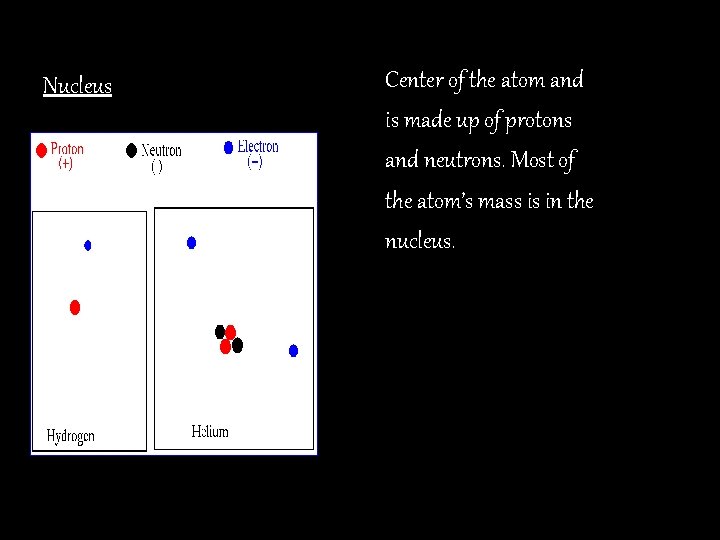
Nucleus Center of the atom and is made up of protons and neutrons. Most of the atom’s mass is in the nucleus.

Protons The particles in the nucleus that have a positive charge. (+) Neutrons Particles in the nucleus with a neutral or no charge. Electrons Are located around the edge of the atom and have a negative charge. (-) They can be easily removed.

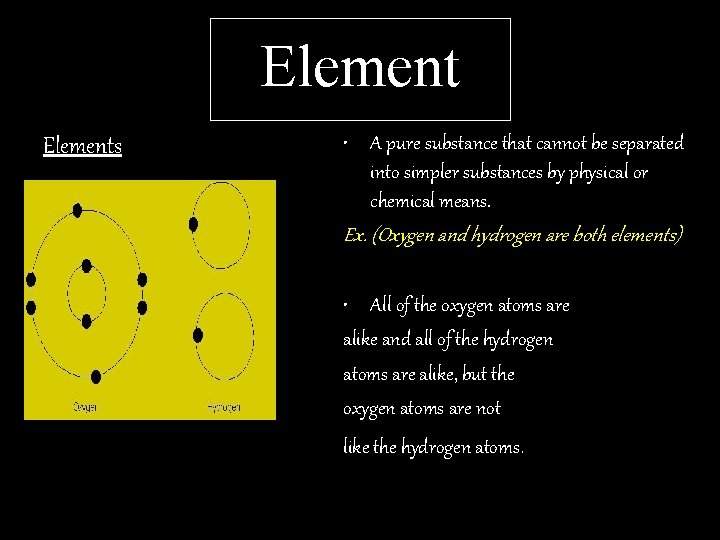
Elements • A pure substance that cannot be separated into simpler substances by physical or chemical means. Ex. (Oxygen and hydrogen are both elements) • All of the oxygen atoms are alike and all of the hydrogen atoms are alike, but the oxygen atoms are not like the hydrogen atoms.

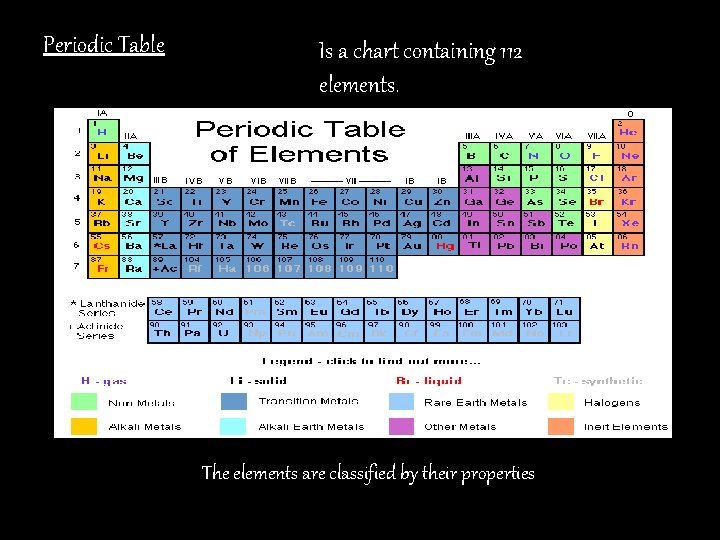
Periodic Table Is a chart containing 112 elements. The elements are classified by their properties

Chemical Symbols Abbreviations for elements O=Oxygen H=Hydrogen See pg. 224 (Old Book)

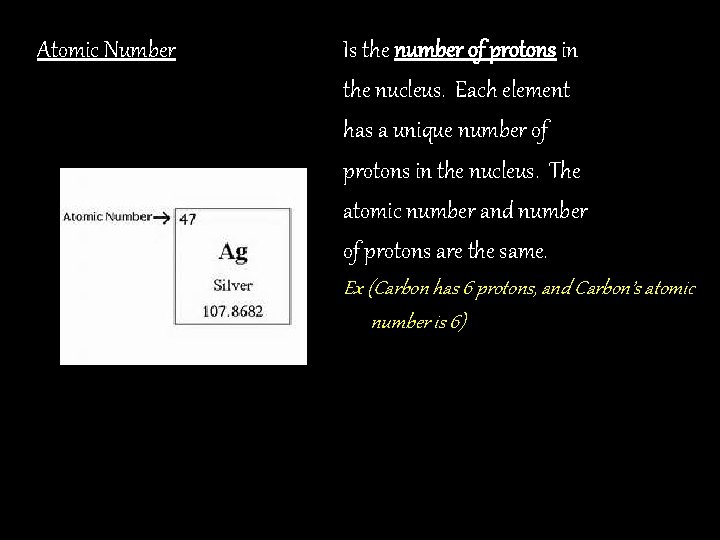
Atomic Number Is the number of protons in the nucleus. Each element has a unique number of protons in the nucleus. The atomic number and number of protons are the same. Ex (Carbon has 6 protons, and Carbon’s atomic number is 6)

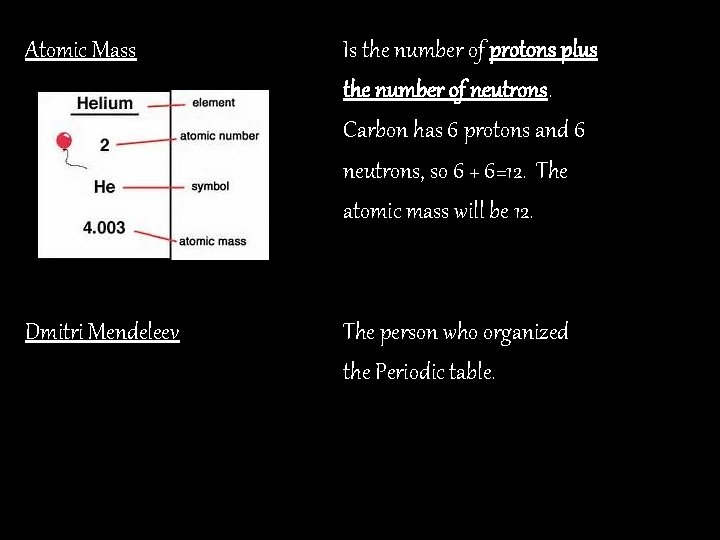
Atomic Mass Is the number of protons plus the number of neutrons. Carbon has 6 protons and 6 neutrons, so 6 + 6=12. The atomic mass will be 12. Dmitri Mendeleev The person who organized the Periodic table.

Compounds • Compounds • A pure substance made of two or more elements that are chemically combined.

Common Compounds Compound Elements Combined Table Salt Sodium & Chlorine Water Hydrogen & Oxygen Carbon Dioxide Carbon & Oxygen Baking Soda Sodium, hydrogen, carbon, & oxygen

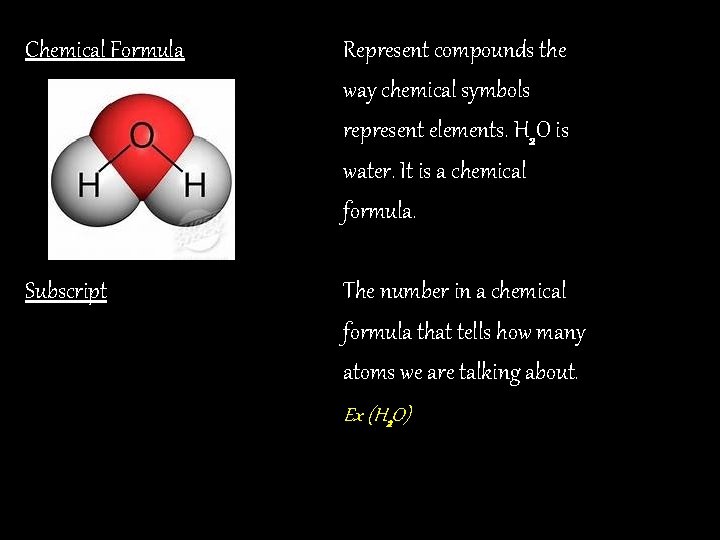
Chemical Formula Represent compounds the way chemical symbols represent elements. H 2 O is water. It is a chemical formula. Subscript The number in a chemical formula that tells how many atoms we are talking about. Ex (H 2 O)

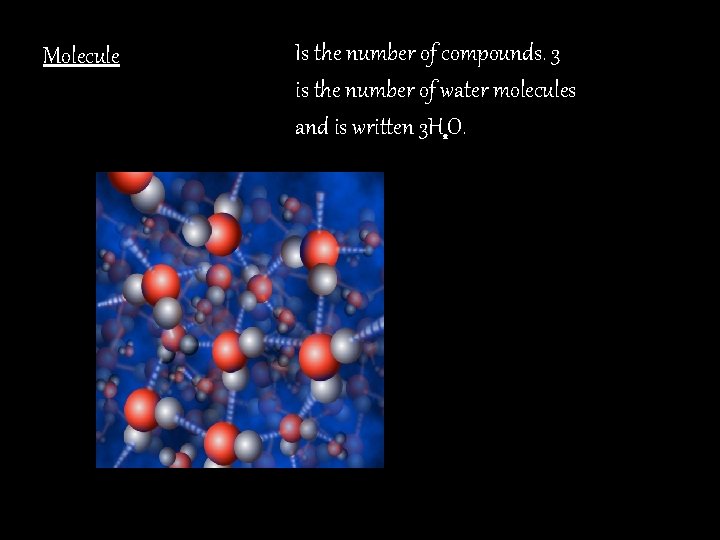
Molecule Is the number of compounds. 3 is the number of water molecules and is written 3 H 2 O.

Physical Change vs. Chemical change

9. 3 Physical and Chemical Changes Physical Change Is a change in size, shape or form. Ex (cutting paper into pieces or changing water to ice) The earth is shaped by physical changes such as weathering when water freezes and then thaws and cracks rocks.

Chemical change • Chemical Change • When one or more substances are changed into new substances and have new and different properties. • Examples: Metal Rusting, Milk souring, fireworks, cooking Digesting your food, Using a battery

Baking a Cake, Chemical or Physical? • You take all of these ingredients, mix them and put them in the oven. =

Indicators of a Chemical Reaction 1. Formation of a Precipitate 2. Gas bubbles are released 3. It changes energy (Heat is added or released) 4. It may change color 5. A change in odor

Formation of a Precipitate There is a formation of a solid when combining two solutions

Bubbles The formation of a gas (bubbles or fizzing) is a clue that a chemical reaction is taking place. Example: Alka-Seltzer dropped in water

Change in Energy (Heat) • A chemical reaction involves the breaking of bonds in the reactants and the forming of bonds in the product. Endothermic (Temperature drops)Absorb more energy than they release. Exothermic (Temperature Increases) Release more energy than they absorb.

Change in Color In a chemical reaction there is often a change in color from the original substance to the new one. (Examples: Rusting, Fireworks, Leaves changing

Change in Odor Chemical reactions may give off a smell or new odor. (Example: Food rotting, such as rotten eggs)

Law of Conservation of Mass or Matter can’t be created or destroyed. It is just converted to another form. Ex. (Burning wood)

Law of Conservation of Matter or Law of Conservation of Mass

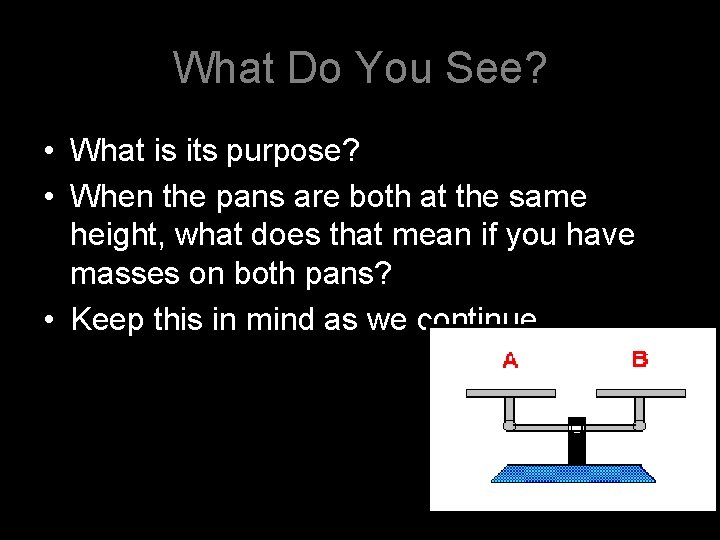
What Do You See? • What is its purpose? • When the pans are both at the same height, what does that mean if you have masses on both pans? • Keep this in mind as we continue

The Law • The law of conservation of matter states that matter (mass) can neither be created nor destroyed. It can, however, can be rearranged. • In a chemical reaction, the mass of the reactants must equal the mass of the products. • HUH?

Thinking of the balance? • Everything must be equal! • Remember the lab we just did? • When matter goes through a physical or chemical change, the amount (or mass) of the substances that you begin with must equal the amount (or mass) of the substances that you end with. • The before and after must balance • Why, you ask? • It’s the Law!!!!

Lab Activity • Let’s take a minute to think • (Demo: Add Sugar to water – determine mass before adding) • Just think about this…what will the mass be when we put it back on the balance? • Look at the before and after data • What do you notice about the sugar and the water? • Were any laws broken? • Explain

Law of Conservation of Matter (Mass) • If the amount of matter (mass) was not the same before and after the change (remember, it is the law), you must offer an explanation as to why.