Chapter 5 The Periodic Law History Cannizzaro 1860







- Slides: 20

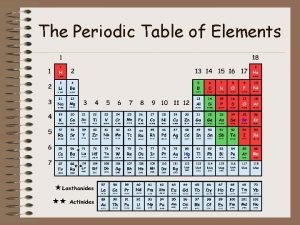
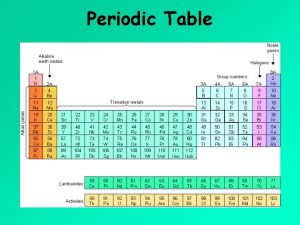
Chapter 5 The Periodic Law

History Cannizzaro- 1860 presented a new method for determining mass of elements gave scientists a way to organize elements

History Mendeleev-Russian 1869 organized elements according to mass and properties noticed that properties repeated periodically predicted elements, later identified as Sc, Ga, Ge

History Moseley-1911 Used X-rays for determining the # of protons This is how our current periodic table is arranged.

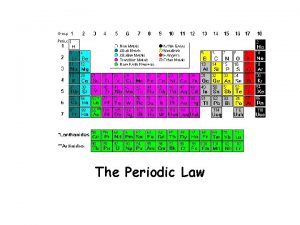
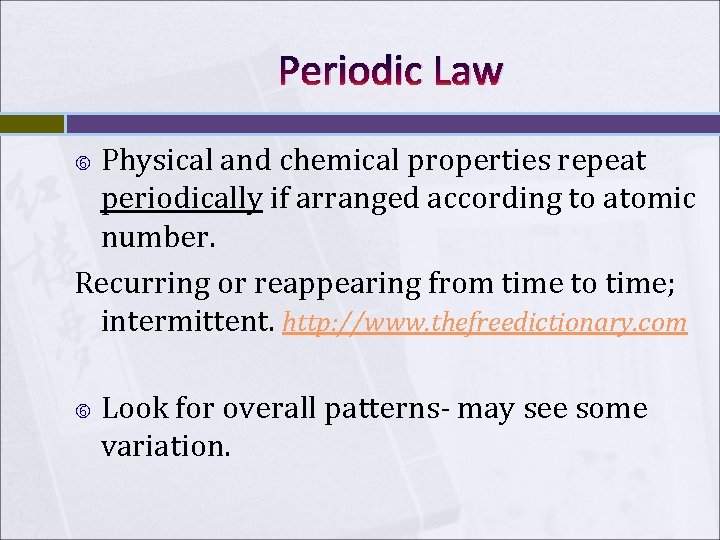
Periodic Law Physical and chemical properties repeat periodically if arranged according to atomic number. Recurring or reappearing from time to time; intermittent. http: //www. thefreedictionary. com Look for overall patterns- may see some variation.

History Changes since Moseley Ø Ø Noble gases-Ramsay Lanthanides/Actinides- Seaborg


The Periodic Table TRENDS

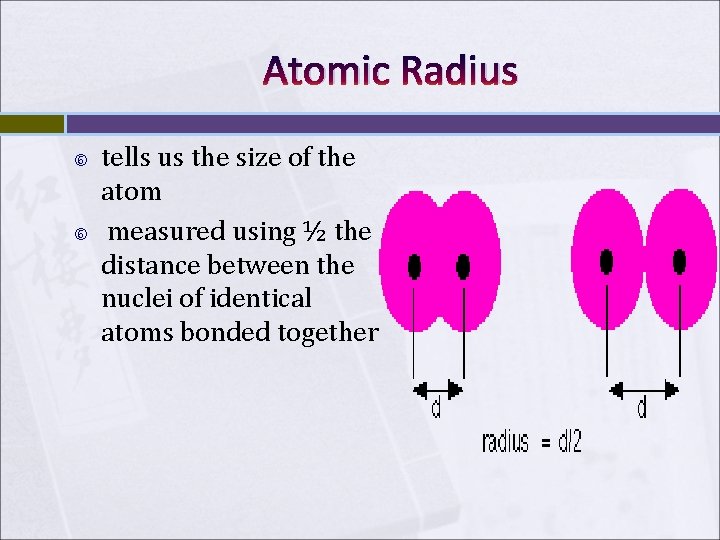
Atomic Radius tells us the size of the atom measured using ½ the distance between the nuclei of identical atoms bonded together

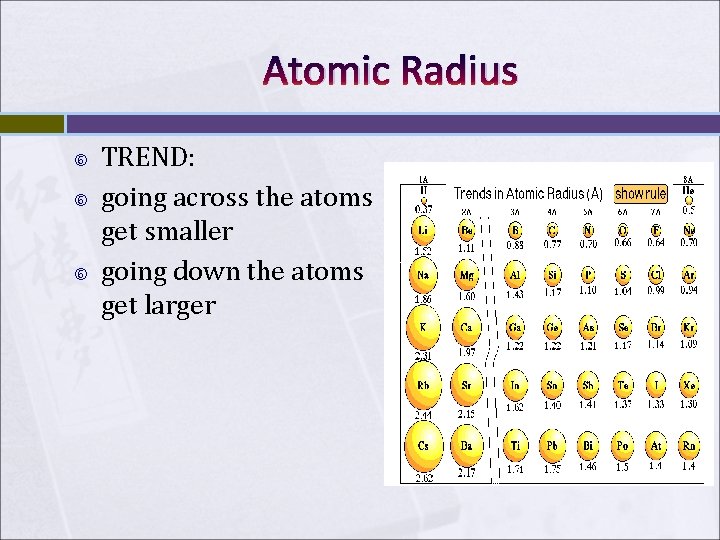
Atomic Radius TREND: going across the atoms get smaller going down the atoms get larger

Ionic Radius tells us the size of the ion Ø positive or negative atom because of loss or gain of electrons Cation-positive ion Anion-negative ion

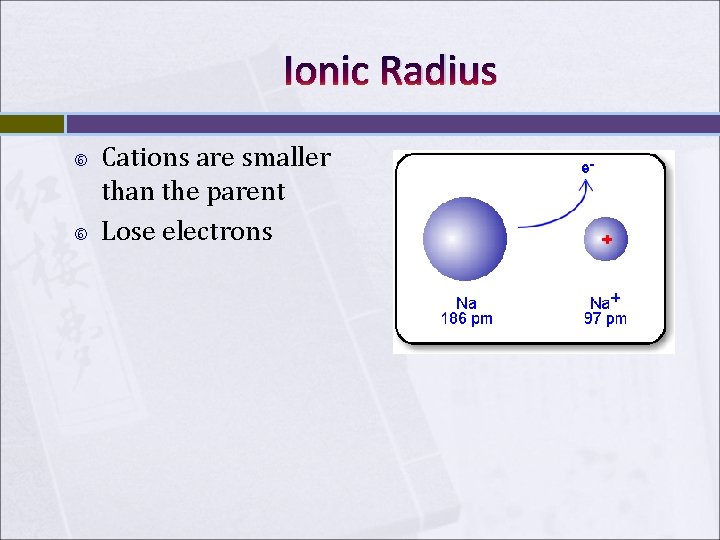
Ionic Radius Cations are smaller than the parent Lose electrons

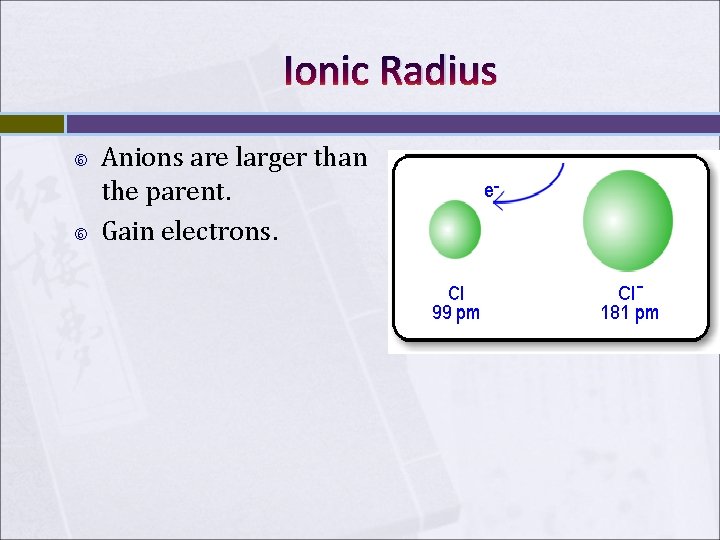
Ionic Radius Anions are larger than the parent. Gain electrons.

Ion Formation Why do ions form? Ø Ø Ø Atoms try to become stable. Achieve a noble gas configuration. Become isoelectronic with noble gases Having the same electron configuration

Ion Formation Examples: Ø Calcium Ø Nitrogen

Ion Formation You try these: Ø Potassium Ø Iodine Ø Aluminum

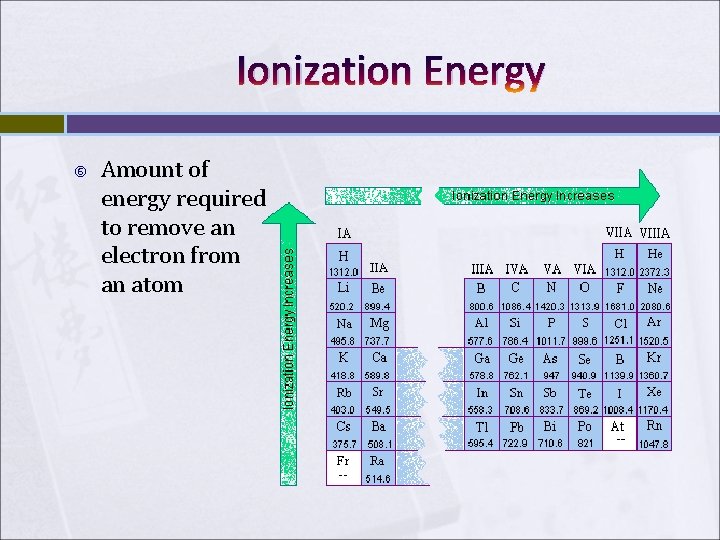
Ionization Energy Amount of energy required to remove an electron from an atom

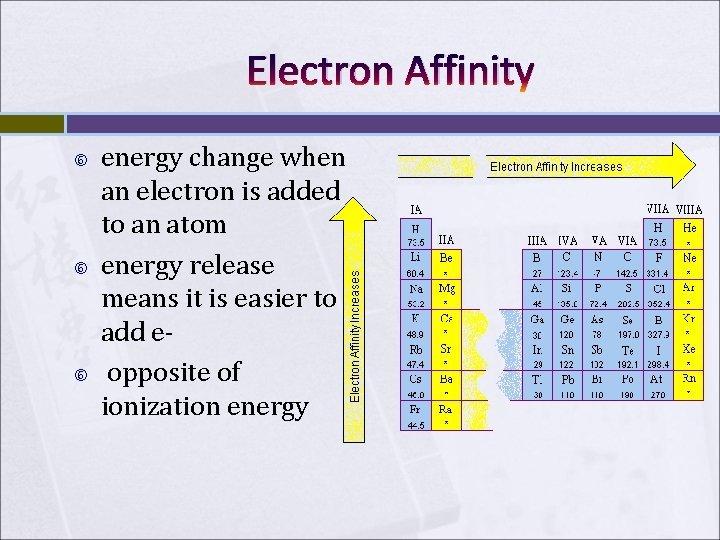
Electron Affinity energy change when an electron is added to an atom energy release means it is easier to add e opposite of ionization energy

Electronegativity measure of the ability of an atom to attract electrons when it is bonded to another atom Think: sharing with a partner, equal sharing or unequal sharing

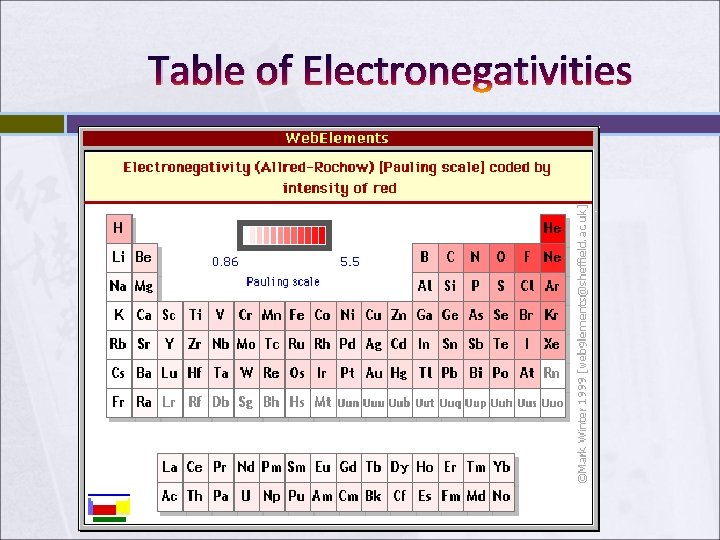
Table of Electronegativities
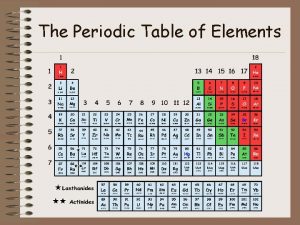
 Chapter 6 the periodic table
Chapter 6 the periodic table 6 the periodic table
6 the periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Mecanismo de la reaccion de cannizzaro con benzaldehido
Mecanismo de la reaccion de cannizzaro con benzaldehido Addition elimination reaction
Addition elimination reaction Cannizzaro reaction
Cannizzaro reaction Filosofo siciliano
Filosofo siciliano Chapter 12: religion, romanticism, and reform, 1800–1860
Chapter 12: religion, romanticism, and reform, 1800–1860 Chapter 5 section 1 history of the periodic table
Chapter 5 section 1 history of the periodic table Border states in 1860
Border states in 1860 Daniel hess aspirateur
Daniel hess aspirateur Romantic period in american literature
Romantic period in american literature Election of 1860 definition
Election of 1860 definition Apush 1820 to 1860
Apush 1820 to 1860 Era of the democrats 1800-1860
Era of the democrats 1800-1860 Decreto 1860 de 1994
Decreto 1860 de 1994 American romanticism 1800 to 1860 worksheet answers
American romanticism 1800 to 1860 worksheet answers South carolina 1860
South carolina 1860 1860
1860 American romanticism 1800 to 1860 worksheet answers
American romanticism 1800 to 1860 worksheet answers Rationalism vs romanticism
Rationalism vs romanticism