Chapter 43 Restorative and Esthetic Dental Materials Copyright

















































- Slides: 52

Chapter 43 Restorative and Esthetic Dental Materials Copyright 2003, Elsevier Science (USA). All rights reserved. No part of this product may be reproduced or transmitted in any form or by any means, electronic or mechanical, including input into or storage in any information system, without permission in writing from the publisher. Power. Point® presentation slides may be displayed and may be reproduced in print form for instructional purposes only, provided a proper copyright notice appears on the last page of each print-out. Produced in the United States of America ISBN 0 -7216 -9770 -4 Copyright 2003, Elsevier Science (USA). All rights reserved.

Introduction Restorative dental materials fulfill an important role in the way dentistry is delivered today. Copyright 2003, Elsevier Science (USA). All rights reserved.

Standardization of Dental Materials § ADA: criteria for a new material • Must not be poisonous or harmful to the body. • Must not be harmful or irritating to the tissues of the oral cavity. • Must help protect the tooth and oral tissues of the oral cavity. • Must resemble the natural dentition. • Must be easily formed and placed in the mouth. Copyright 2003, Elsevier Science (USA). All rights reserved.

Properties of Dental Materials § Mechanical properties must withstand the § § § biting and chewing force in the posterior area of the mouth. Force is any push or pull on matter. Stress is the reaction within the material that can cause distortion. Strain is the change produced within the material that occurs as the result of stress. Copyright 2003, Elsevier Science (USA). All rights reserved.

Mechanical Properties § Types of stress and strain: • Tensile stress pulls and stretches the material. • Compressive stress pushes the material together. • Shear stress is the breakdown of the material. Copyright 2003, Elsevier Science (USA). All rights reserved.

Thermal Changes § A change in temperature in the oral cavity due § to either a hot or cold product. Contraction and expansion • Dental materials will contract or expand at their own rate. • Change in temperature can cause a dental material to pull away from the tooth. • Microleakage • Faulty restoration Copyright 2003, Elsevier Science (USA). All rights reserved.

Electrical Properties § An electrical current, or galvanic action, is § created when two different or dissimilar metals are present in the oral cavity. Conditions: • Saliva. • Two metallic components of different composition. • Electrical current. • Galvanic action, or shock, is the coming together of all conditions. Copyright 2003, Elsevier Science (USA). All rights reserved.

Corrosive Properties § Reaction a metal has when it comes into § contact with corrosive products. Solubility is the degree to which a substance will dissolve in a given amount of another substance. Copyright 2003, Elsevier Science (USA). All rights reserved.

Application Properties § Flow: • The dental material must be pliable enough to be placed in the preparation. § Adhesion: • The force that causes unlike materials to adhere to each other. § Wetting is the ability of a liquid to flow over the surface. § Viscosity is the property of a liquid that causes it not to flow easily. Copyright 2003, Elsevier Science (USA). All rights reserved.

Application Properties- cont’d § Surface characteristics is where a liquid flows § § § more easily on a rough surface than on a very smooth surface. Film thickness: In general, the thinner the film, the stronger the adhesive junction. Retention is the ability to hold two things firmly together when they will not adhere to each other. Curing • Auto-cured material hardens as the result of a chemical reaction of the materials. • Light-cured material does not harden until it has been exposed to a curing light. Copyright 2003, Elsevier Science (USA). All rights reserved.

Restorative and Esthetic Materials § Restorative: To replace or bring something § back to its natural appearance and function. Esthetic: To replace or bring something back to its pleasing appearance. Copyright 2003, Elsevier Science (USA). All rights reserved.

Direct Restorations § Restorative materials that are applied to the tooth while the material is pliable and able to carve and finish. • Amalgam • Composite resins • Glass ionomer • Intermediate restorative materials • Tooth-whitening products Copyright 2003, Elsevier Science (USA). All rights reserved.

Amalgam § Amalgam is a safe, affordable, and durable material that is used predominantly to restore premolars and molars (Figure 43 -8). Copyright 2003, Elsevier Science (USA). All rights reserved.

Fig. 43 -8 Packing an amalgam carrier. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using Amalgam § In individuals of all ages. § In stress-bearing areas of the mouth. § When there is severe destruction of tooth § § structure. As a foundation. When personal oral hygiene is poor. When moisture control is problematic. When cost is an overriding patient concern. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Not Using Amalgam § Esthetics is important. § Patient has a history of allergy to mercury or § other amalgam components. The cost of other restorative materials or treatment options is not a factor. Copyright 2003, Elsevier Science (USA). All rights reserved.

Chemical Makeup of Amalgam § Mercury (43% to 54%) § Alloy powder (57% to 46%) • Silver, which gives it its strength. • Tin for its workability and strength. • Copper for its strength and corrosion resistance. • Zinc to suppress oxidation. Copyright 2003, Elsevier Science (USA). All rights reserved.

Issues Concerning Amalgam § Harm to patients: Essentially harmless. § The exception is with patients who have many § amalgam restorations, or a high sensitivity to metals. Harm to Dental Personnel: Health concerns with high exposure to mercury, not amalgam. • Tremors • Kidney dysfunction • Depression • Nervous system disorders Copyright 2003, Elsevier Science (USA). All rights reserved.

Amalgam Hygiene § § § Do not contact mercury with your skin. Protect against spillage during trituration. Keep lid closed during trituration. Do not discard scrap amalgam into waste containers. Collect all scrap amalgam and store under water or photographic fixer solutions in a closed container. Copyright 2003, Elsevier Science (USA). All rights reserved.

Preparation of Amalgam § Capsules (600 mg of alloy): For small or § § single‑surface restorations. Capsules (800 mg of alloy): For larger restorations. Trituration: The process by which the mercury and alloy are mixed together to form the mass of amalgam. Copyright 2003, Elsevier Science (USA). All rights reserved.

Direct Application of Amalgam 1. Mixed amalgam placed in amalgam well. 2. Amalgam carried to the prepared tooth. 3. Amalgam placed in increments in the 4. 5. 6. 7. prepared tooth. Each increment is condensed immediately. Carvers are used to carve anatomy into the amalgam. A burnisher is used to smooth the amalgam. The new restorations occlusion is checked. Copyright 2003, Elsevier Science (USA). All rights reserved.

Composite Resins § Becoming the most widely accepted material of choice by dentists and patients because of their esthetic qualities and new advances in their strength (Figure 43 -13). Copyright 2003, Elsevier Science (USA). All rights reserved.

Fig. 43 -13 Resins supplied in a syringe. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using Composite Resins § § Withstand the environments of the oral cavity. Be easily shaped to the anatomy of a tooth. Match the natural tooth color. Be bonded directly to the tooth surface. Copyright 2003, Elsevier Science (USA). All rights reserved.

Chemical Makeup of Composite Resins § Resin matrix • Dimethacrylate, referred to as BIS‑GMA • Monomer used to make synthetic resins • Polymerization additives • Allow the material to take form through a chemical process • Initiator • Accelerator • Retarder • Ultraviolet (UV) stabilizers Copyright 2003, Elsevier Science (USA). All rights reserved.

Chemical Makeup of Composite Resins-cont’d § Fillers Add the strength and characteristics § necessary for use as a restorative material. Inorganic fillers • Quartz • Glass • Silica • Colorants Copyright 2003, Elsevier Science (USA). All rights reserved.

Chemical Makeup of Composite Resins-cont’d § A coupling agent strengthens the resin by chemically bonding the filler to the resin matrix. • Organosilane compound Copyright 2003, Elsevier Science (USA). All rights reserved.

Types of Composites § Macrofilled composites contain the largest of § § filler particles, providing greater strength but a duller, rougher surface. Microfilled composites: The inorganic filler is much smaller and is capable of producing a highly polishee, finished restoration, which is used primarily in anterior restoration. Hybrid composites contain both macrofill and microfill particles. Copyright 2003, Elsevier Science (USA). All rights reserved.

Polymerization of Composite Resins § The process in which the resin material is changed from a plastic state into a hardened restoration. • Auto-Cured • Light-Cured Copyright 2003, Elsevier Science (USA). All rights reserved.

Direct Application of Composite Resins 1. Select the shade of the tooth. 2. Express the needed amount of material onto 3. 4. 5. the treated pad or in the light-protected well. Material placed in increments. Material is light-cured. Material is finished and polished. Copyright 2003, Elsevier Science (USA). All rights reserved.

Steps in Finishing a Composite Restoration 1. Reduction of the material is completed by 2. 3. 4. 5. the use of a white stone or a finishing diamond. Fine finishing is completed with carbide finishing burs and diamond burs. Polish with medium discs and finish with the superfine discs. Finishing strips assist in the polishing of the interproximal surfaces. Use polishing paste with a rubber cup. Copyright 2003, Elsevier Science (USA). All rights reserved.

Glass Ionomer Materials § Glass ionomer is a versatile material with chemical properties allowing it to be a restorative material, liner, bonding agent, and permanent cement. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using Glass Ionomers § § § Primary teeth. Final restorations in non-stress areas. Intermediate restorations. Core material for a buildups. Long-term temporary restorations. Copyright 2003, Elsevier Science (USA). All rights reserved.

Qualities of Glass Ionomers § The ability to chemically bind to the teeth. § No need to prepare the tooth structure as § extensively as for preparing for an amalgam or composite resin. The release of fluoride after its final setting. Copyright 2003, Elsevier Science (USA). All rights reserved.

Properties of Glass Ionomers § Glass Ionomer § Glass § § • Ceramic particles • Glassy matrix Acrylic acid Tartaric acid Maleic acid Metal-reinforced glass ionomer • Silver-tin alloy + Glass ionomer Copyright 2003, Elsevier Science (USA). All rights reserved.

Supply of Glass Ionomers § Powder and Liquid: Manually mixed together § § § on a treated paper pad. Light-Protected Tubes: Dispensed onto a treated paper pad. Paste/Paste System: Mixed for application. Premeasured Capsule: Triturated for application. Copyright 2003, Elsevier Science (USA). All rights reserved.

Temporary Restorative Materials § Designed to maintain or restore function to a tooth or teeth and keep the patient comfortable for a period of time. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using a Temporary Restorative Material § Reduce sensitivity and discomfort of a tooth to § § § determine its diagnosis. Maintain the function and esthetics of a tooth until a permanent restoration can be placed. Protect the margins of a prepared tooth that will receive a permanent casting at a later time. Prevent shifting of the adjacent or opposing teeth because of open space. Copyright 2003, Elsevier Science (USA). All rights reserved.

Intermediate Restorative Materials (IRM) § Composition: • Zinc-Oxide gives strength and durability. • Eugenol has a sedative effect. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using IRM § § Restoration of primary teeth Restorative emergencies Caries management program Supply of IRM • Powder/liquid • Premeasured capsules Copyright 2003, Elsevier Science (USA). All rights reserved.

Provisional Restorative Materials § Restorative material that covers the major portion, if not the entire clinical portion of a tooth or several teeth for a period of time. Copyright 2003, Elsevier Science (USA). All rights reserved.

Types of Materials Used § Auto-cured acrylic (methylmethacrylate) § Light-cured resin § Process of application • Material is placed in either an alginate impression or a vacuum-formed tray. • Material is seated over the prepared tooth and allowed to cure. • Occlusion is adjusted. • Material is cemented in place with temporary cement. Copyright 2003, Elsevier Science (USA). All rights reserved.

Tooth Whitening Materials § The process of applying a material on anterior teeth for a prescribed period of time to whiten the color of one’s teeth. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using Tooth-Whitening Products § § § § Teeth discolored Aging Consumption of staining substances Trauma Tetracycline staining Excessive fluoride Nerve degeneration Old restorations Copyright 2003, Elsevier Science (USA). All rights reserved.

Tooth-Whitening Products § Carbamide Peroxide: When the carbamide § peroxide breaks down, oxygen enters the enamel and dentin and bleaches the colored substances. Concentrations: 10%, 16%, 22% Copyright 2003, Elsevier Science (USA). All rights reserved.

Indirect Restorations § Types of dental restorations that dental § laboratory technicians create in the dental laboratory. These restorations are also referred to as castings, cannot be reshaped, and are carved once they are in this stage. Copyright 2003, Elsevier Science (USA). All rights reserved.

Gold Alloys § By combining gold with other metals to form an alloy, it creates the characteristics and hardness required as an excellent choice for an indirect restoration. • Gold • Palladium • Platinum Copyright 2003, Elsevier Science (USA). All rights reserved.

Types of Casting Alloys § Soft, Type I alloys are used for casting inlays § § § subject to slight stress during mastication. Medium, Type II alloys can be used for practically all types of cast inlays and possibly posterior bridge abutments. Hard, Type III alloys are acceptable for inlays, full crowns, three‑quarter crowns, and anterior or posterior bridge abutments. Extra-hard, Type IV alloys are designed for cast-removable partial dentures. Copyright 2003, Elsevier Science (USA). All rights reserved.

Ceramics § Ceramics are compounds that involve a combination of metallic and nonmetallic elements, creating strength and aesthetics. Copyright 2003, Elsevier Science (USA). All rights reserved.

Types of Ceramic Restorations § § Porcelain fused to metal (PFM) Porcelain bonded to metal (PBM) Ceramco-metal restorations Porcelain-metal restorations (P-M) Copyright 2003, Elsevier Science (USA). All rights reserved.

Porcelain § Type of ceramic that is most commonly used in dentistry. It combines strength, translucence and the ability to match the natural tooth color. Copyright 2003, Elsevier Science (USA). All rights reserved.

Indications for Using Porcelain § The shading of colors matches the tooth color § § well. It esthetically improves the appearance of anterior teeth. It has the strength of metal. The material is a good insulator. The material has a low coefficient of thermal expansion. Copyright 2003, Elsevier Science (USA). All rights reserved.
 Somesthetic senses definition
Somesthetic senses definition Restorative dental instruments
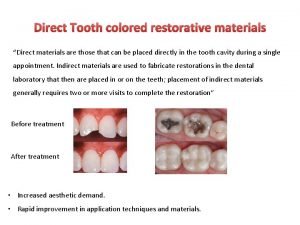
Restorative dental instruments Tooth colored restorative materials
Tooth colored restorative materials 21 rehabilitation and restorative care
21 rehabilitation and restorative care Chapter 41 rehabilitation and restorative nursing care
Chapter 41 rehabilitation and restorative nursing care Chapter 21 rehabilitation and restorative care
Chapter 21 rehabilitation and restorative care 21 rehabilitation and restorative care
21 rehabilitation and restorative care Physical properties of dental material
Physical properties of dental material Matrix band definition
Matrix band definition Chapter 49 matrix systems for restorative dentistry
Chapter 49 matrix systems for restorative dentistry Cant stop the feeling go noodle
Cant stop the feeling go noodle Materials useful or harmful
Materials useful or harmful Natural materials and man made materials
Natural materials and man made materials Adopting materials
Adopting materials Preventive dental materials
Preventive dental materials Polymers in dental materials
Polymers in dental materials Resilience dental materials
Resilience dental materials Ideal requirements of restorative material
Ideal requirements of restorative material Auxiliary dental material
Auxiliary dental material Most biocompatible dental materials
Most biocompatible dental materials Impression materials in dentistry ppt
Impression materials in dentistry ppt Auxiliary dental materials
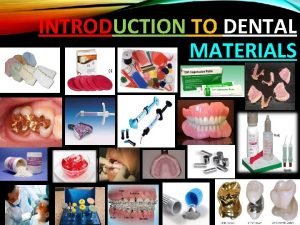
Auxiliary dental materials Introduction to dental materials
Introduction to dental materials Materials department
Materials department Pros and cons of restorative justice
Pros and cons of restorative justice Pbis and restorative practices
Pbis and restorative practices Restorative care definition
Restorative care definition Direct materials budget with multiple materials
Direct materials budget with multiple materials Restorative circle questions
Restorative circle questions Affective statements poster
Affective statements poster Restorative language
Restorative language Principle of restorative justice
Principle of restorative justice Restorative justice powerpoint template
Restorative justice powerpoint template Restorative practice circle questions
Restorative practice circle questions Ali hearn
Ali hearn Restorative conversation script
Restorative conversation script Anita wadhwa
Anita wadhwa Social justice and community action
Social justice and community action San diego restorative justice
San diego restorative justice Punitive vs restorative discipline
Punitive vs restorative discipline Sfusd restorative practices
Sfusd restorative practices San francisco unified school district restorative practices
San francisco unified school district restorative practices Restorative justice hubs chicago
Restorative justice hubs chicago Sycamore tree victim awareness programme
Sycamore tree victim awareness programme Restorative mini cf
Restorative mini cf Peter woolf restorative justice
Peter woolf restorative justice Restorative justice script
Restorative justice script Equilibrium restorative balance
Equilibrium restorative balance Restorative care versus rehabilitation
Restorative care versus rehabilitation Social discipline window
Social discipline window Restorative questions
Restorative questions Social discipline window
Social discipline window Restorative practices
Restorative practices