Chapter 3 Water and Fitness in the Environment






![Fig. 3 -UN 5 + 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Fig. 3 -UN 5 + 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–]](https://slidetodoc.com/presentation_image_h2/83b05069f0f2d49d6a02cd060bb54e10/image-26.jpg)



- Slides: 30

+ Chapter 3: Water and Fitness in the Environment AP Biology Mrs. Madalon

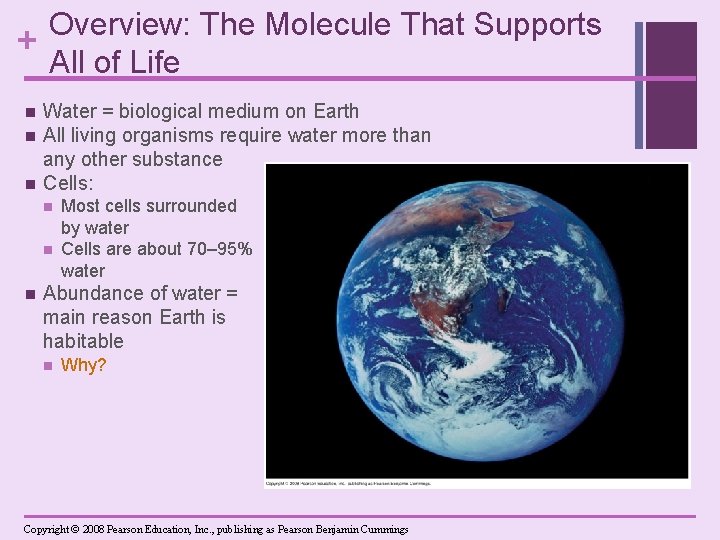
Overview: The Molecule That Supports + All of Life n n n Water = biological medium on Earth All living organisms require water more than any other substance Cells: n n n Most cells surrounded by water Cells are about 70– 95% water Abundance of water = main reason Earth is habitable n Why? Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

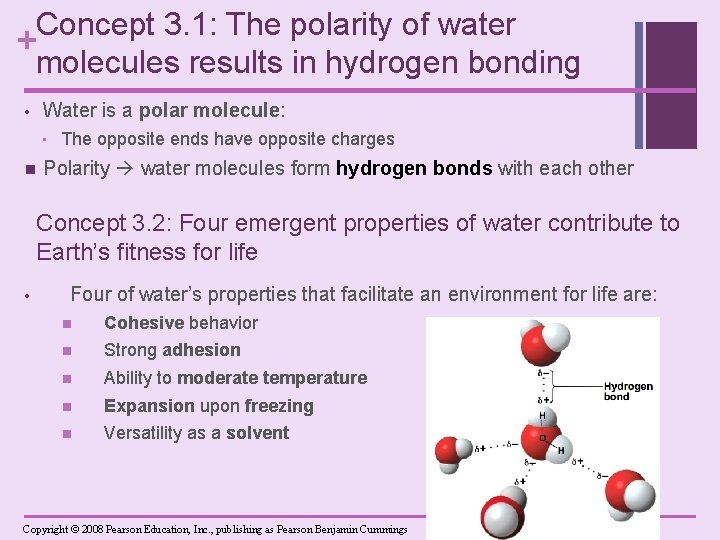
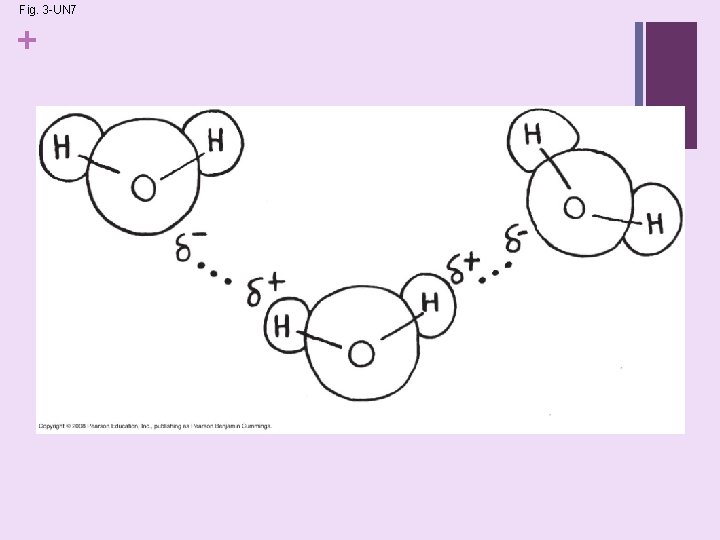
Concept 3. 1: The polarity of water + molecules results in hydrogen bonding Water is a polar molecule: • • n The opposite ends have opposite charges Polarity water molecules form hydrogen bonds with each other Concept 3. 2: Four emergent properties of water contribute to Earth’s fitness for life • Four of water’s properties that facilitate an environment for life are: n Cohesive behavior n Strong adhesion n Ability to moderate temperature n Expansion upon freezing n Versatility as a solvent Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

Fig. 3 -UN 7 +

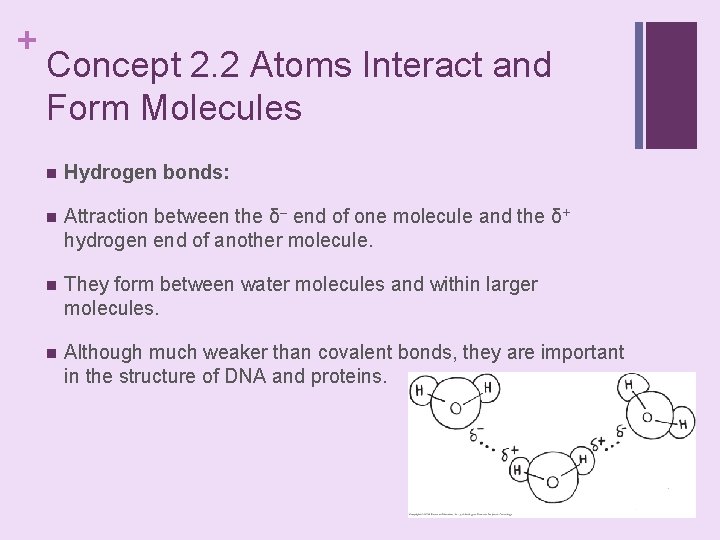
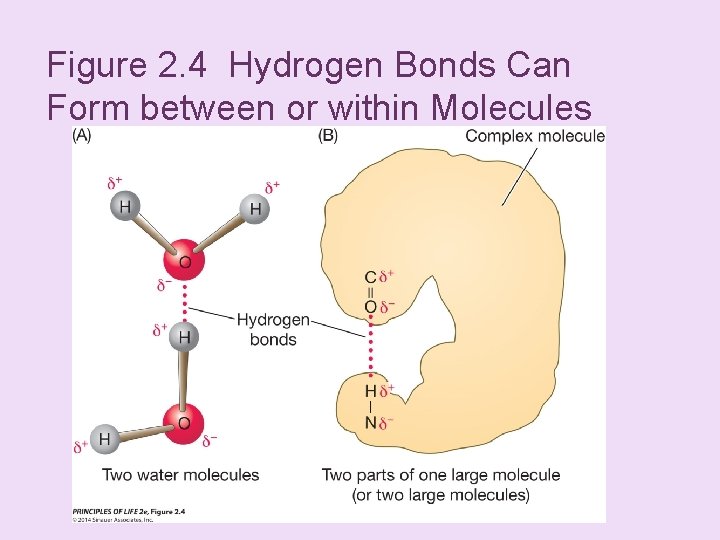
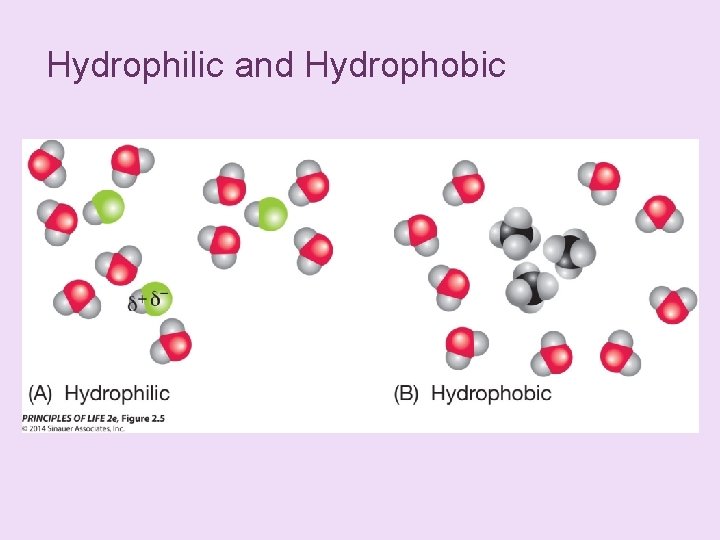
+ Concept 2. 2 Atoms Interact and Form Molecules n Hydrogen bonds: n Attraction between the δ– end of one molecule and the δ+ hydrogen end of another molecule. n They form between water molecules and within larger molecules. n Although much weaker than covalent bonds, they are important in the structure of DNA and proteins.

Figure 2. 4 Hydrogen Bonds Can Form between or within Molecules

+ Water has strong cohesion & high surface tension n Cohesion: Hydrogen bonds hold water molecules together n n helps the transport of water against gravity in plants Surface tension: measure of how hard it is to break the surface of a liquid; related to cohesion Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings


+ Water has strong adhesion n Adhesion: attraction between unlike substances n water and plant cell walls Capillary Action: this adhesive property allows H 2 O to rise against the force of gravity. • moves H 2 O in plants

+ Moderation of Temperature n Water absorbs heat from warmer air; releases stored heat to cooler air n Kinetic energy: the energy of motion Heat: measure of the total amount of kinetic energy due to molecular motion Temperature: measures the intensity of heat due to the average kinetic energy of molecules Celsius scale: measure of temperature using Celsius degrees (°C) Calorie (cal): amount of heat required to raise the temperature of 1 g of water by 1°C • • n n n “Calories” on food packages are actually kilocalories (kcal) n n 1 kcal = 1, 000 cal Joule (J): another unit of energy n 1 J = 0. 239 cal, or 1 cal = 4. 184 J Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

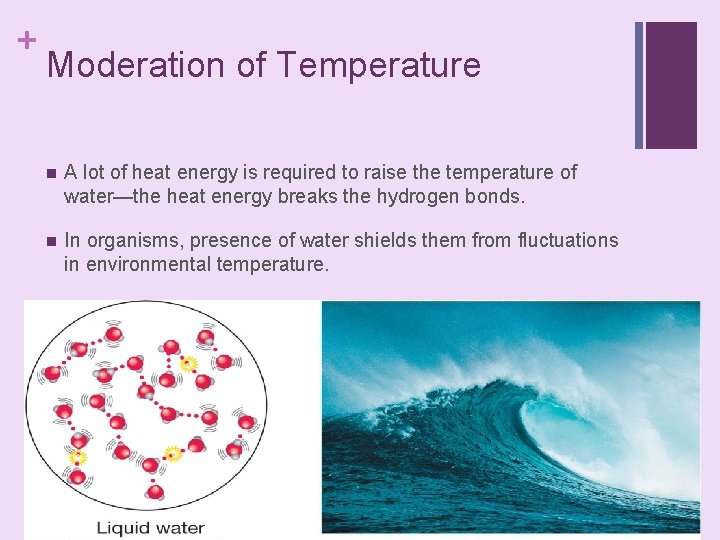
+ Moderation of Temperature n A lot of heat energy is required to raise the temperature of water—the heat energy breaks the hydrogen bonds. n In organisms, presence of water shields them from fluctuations in environmental temperature.

+ Water’s High Specific Heat n n Specific heat: the degree to which a substance changes temperature in response to a gain or loss of heat. Water’s high specific heat resists changing temperature better for LIFE water’s high specific heat n Hydrogen bonding n Heat absorbed when hydrogen bonds break n Heat is released when hydrogen bonds form Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

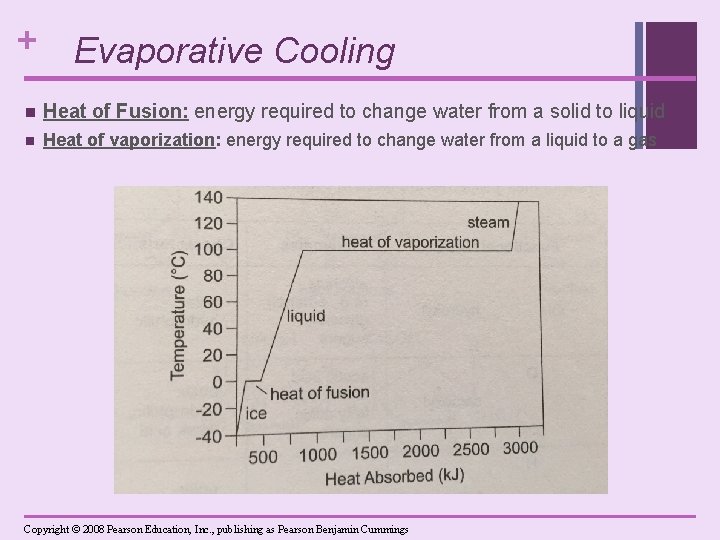
+ Evaporative Cooling n Evaporation: liquid gas n Water has a high heat of vaporization: a lot of heat energy is required to change water from the liquid to gaseous state (to break the hydrogen bonds). n Thus, evaporation has a cooling effect on the environment. [Evaporative Cooling] n Sweating cools the body—as sweat evaporates from the skin, a large amount of heat is taken with it and you are cooled. n helps stabilize temperatures in organisms and bodies of water

+ Evaporative Cooling n Heat of Fusion: energy required to change water from a solid to liquid n Heat of vaporization: energy required to change water from a liquid to a gas Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

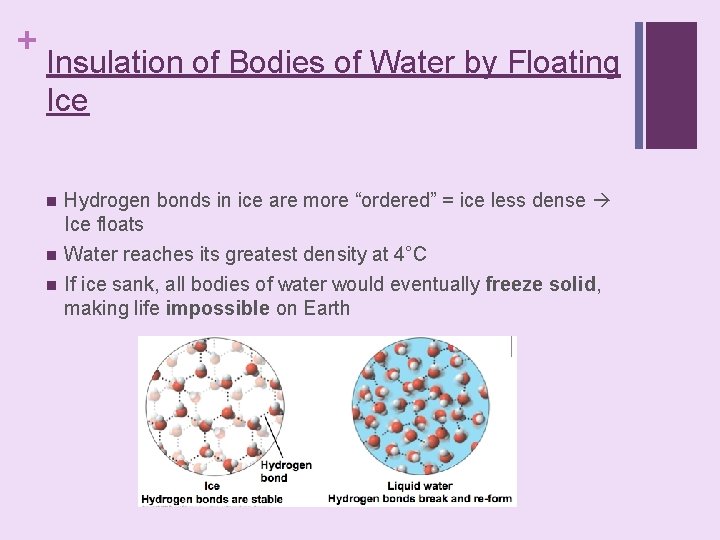
+ Insulation of Bodies of Water by Floating Ice n n n Hydrogen bonds in ice are more “ordered” = ice less dense Ice floats Water reaches its greatest density at 4°C If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth

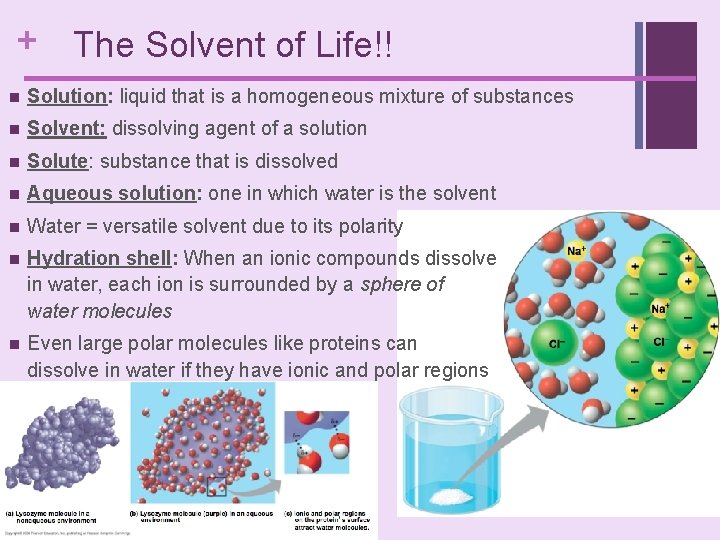
+ The Solvent of Life!! n Solution: liquid that is a homogeneous mixture of substances n Solvent: dissolving agent of a solution n Solute: substance that is dissolved n Aqueous solution: one in which water is the solvent n Water = versatile solvent due to its polarity n Hydration shell: When an ionic compounds dissolve in water, each ion is surrounded by a sphere of water molecules n Even large polar molecules like proteins can dissolve in water if they have ionic and polar regions

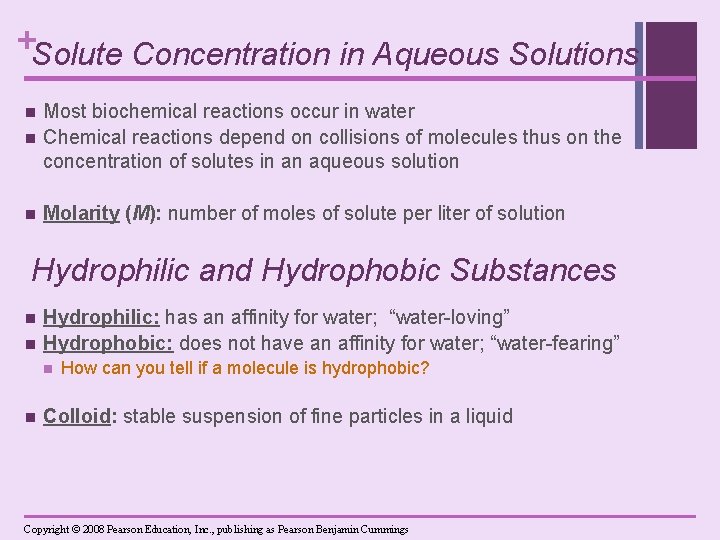
+Solute Concentration in Aqueous Solutions n Most biochemical reactions occur in water Chemical reactions depend on collisions of molecules thus on the concentration of solutes in an aqueous solution n Molarity (M): number of moles of solute per liter of solution n Hydrophilic and Hydrophobic Substances n n Hydrophilic: has an affinity for water; “water-loving” Hydrophobic: does not have an affinity for water; “water-fearing” n n How can you tell if a molecule is hydrophobic? Colloid: stable suspension of fine particles in a liquid Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

+ Hydrophilic and Hydrophobic Substances n n POLAR MOLECULES LIKE EACH OTHER! n Any polar molecule can interact with any other polar molecule through hydrogen bonds. n Hydrophilic (“water-loving”): in aqueous solutions, polar molecules become separated and surrounded by water molecules. Nonpolar molecules are called hydrophobic (“water-hating”); the interactions between them are hydrophobic interactions.

Hydrophilic and Hydrophobic

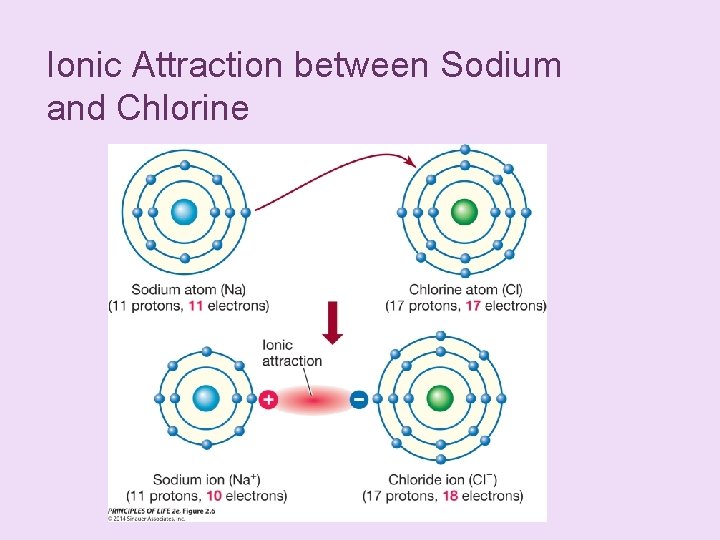
+ Atoms Interact and Form Molecules n When one atom is much more electronegative than the other, a complete transfer of electrons may occur. n n This makes both atoms more stable because their outer shells are full. The result is two ions—electrically charged particles that form when atoms gain or lose one or more electrons.

Ionic Attraction between Sodium and Chlorine

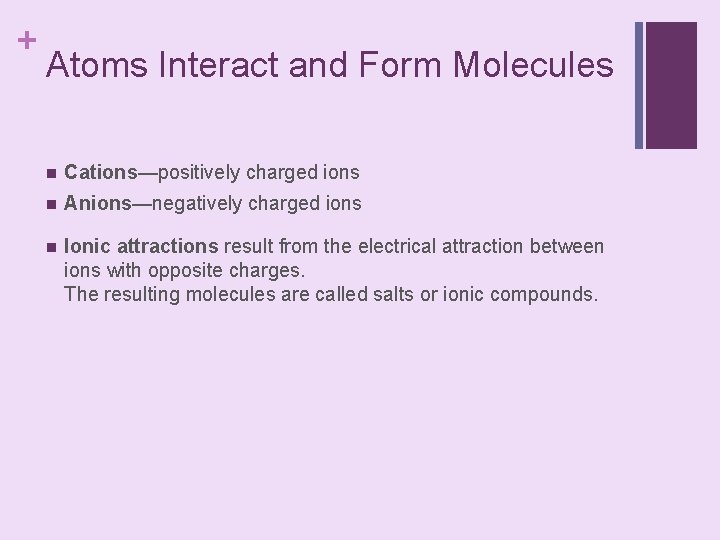
+ Atoms Interact and Form Molecules n Cations—positively charged ions n Anions—negatively charged ions n Ionic attractions result from the electrical attraction between ions with opposite charges. The resulting molecules are called salts or ionic compounds.

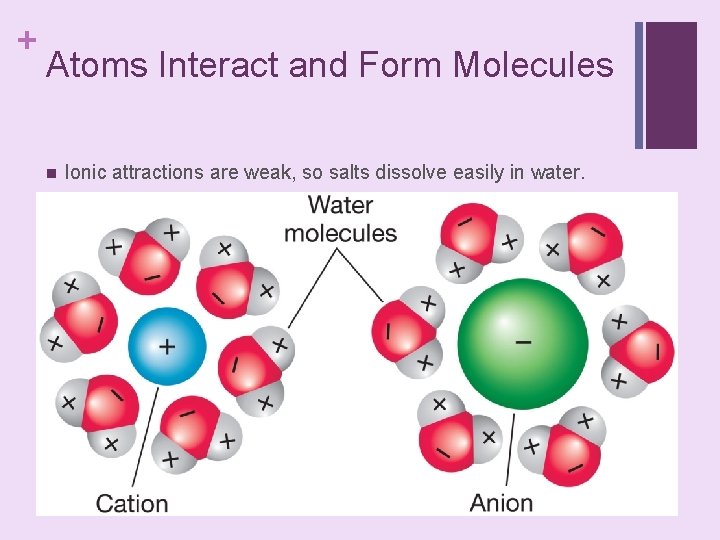
+ Atoms Interact and Form Molecules n Ionic attractions are weak, so salts dissolve easily in water. place text art pg 25 here

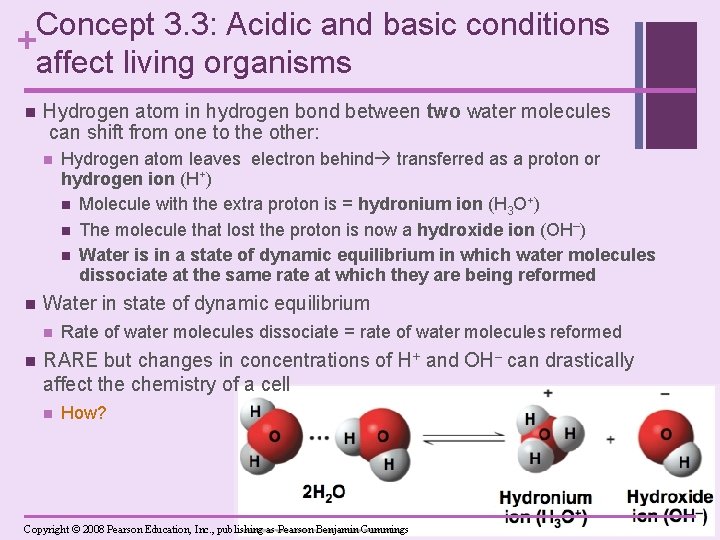
Concept 3. 3: Acidic and basic conditions + affect living organisms n Hydrogen atom in hydrogen bond between two water molecules can shift from one to the other: n n Water in state of dynamic equilibrium n n Hydrogen atom leaves electron behind transferred as a proton or hydrogen ion (H+) n Molecule with the extra proton is = hydronium ion (H 3 O+) n The molecule that lost the proton is now a hydroxide ion (OH–) n Water is in a state of dynamic equilibrium in which water molecules dissociate at the same rate at which they are being reformed Rate of water molecules dissociate = rate of water molecules reformed RARE but changes in concentrations of H+ and OH– can drastically affect the chemistry of a cell n How? Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

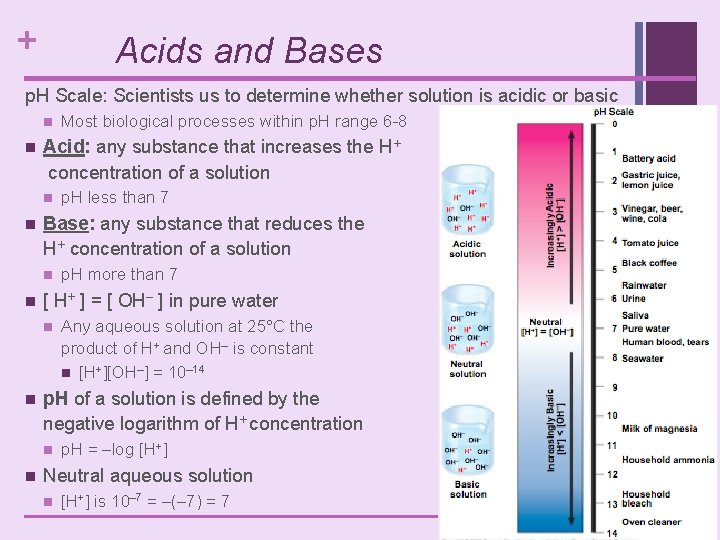
+ Acids and Bases p. H Scale: Scientists us to determine whether solution is acidic or basic n n Acid: any substance that increases the H+ concentration of a solution n n Any aqueous solution at 25°C the product of H+ and OH– is constant n [H+][OH–] = 10– 14 p. H of a solution is defined by the negative logarithm of H+ concentration n n p. H more than 7 [ H+ ] = [ OH– ] in pure water n n p. H less than 7 Base: any substance that reduces the H+ concentration of a solution n n Most biological processes within p. H range 6 -8 p. H = –log [H+] Neutral aqueous solution n [H+] is 10– 7 = –(– 7) = 7
![Fig 3 UN 5 0 Acidic H OH Neutral H OH Fig. 3 -UN 5 + 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–]](https://slidetodoc.com/presentation_image_h2/83b05069f0f2d49d6a02cd060bb54e10/image-26.jpg)
Fig. 3 -UN 5 + 0 Acidic [H+] > [OH–] Neutral [H+] = [OH–] Basic [H+] < [OH–] Acids donate H+ in aqueous solutions 7 Bases donate OH– or accept H+ in aqueous solutions 14

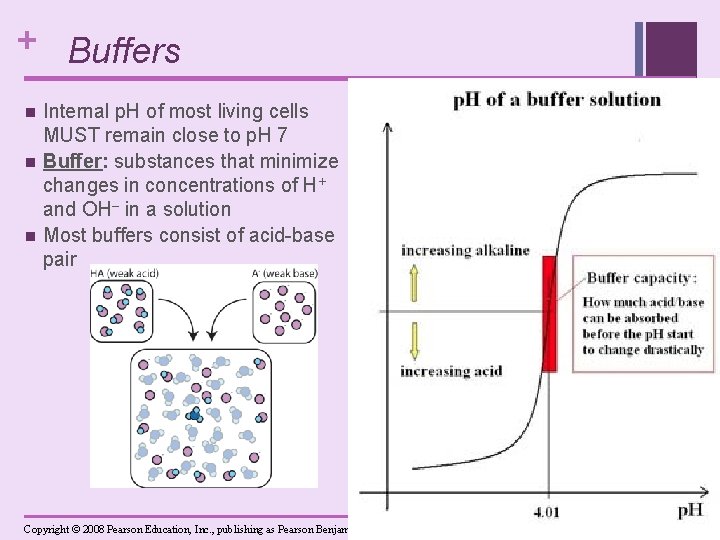
+ Buffers n n n Internal p. H of most living cells MUST remain close to p. H 7 Buffer: substances that minimize changes in concentrations of H+ and OH– in a solution Most buffers consist of acid-base pair Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

+ Threats to Water Quality on Earth n n Acid precipitation: rain, snow, or fog with a p. H lower than 5. 6 n can fall far from pollutant source n can damage life in lakes and streams n Effects on soil chemistry are contribute to decline of some forests Pollutants + water in air = Acid Rain Burning fossil fuels Poor water quality CO 2 is released by fossil fuel combustion: n “Greenhouse effect” n Acidification of the oceans n this decrease in the ability of corals to form calcified reefs Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings

+ WHAT DID WE COVER? 1. Water is necessary for LIFE! 2. Water is polar 3. Water has strong cohesion & high surface tension 4. Water has strong adhesion 5. Water has ability to moderate temperature 6. Water has a high specific heat capacity 1. Evaporative cooling 7. Ice floats- unique density properties 8. Water is the universal solvent 9. Hydrophilic vs. Hydrophobic 10. p. H

+ You should now be able to: 1. List and explain the four properties of water that emerge as a result of its ability to form hydrogen bonds 2. Distinguish between the following sets of terms: hydrophobic and hydrophilic substances; a solute, a solvent, and a solution 3. Define acid, base, and p. H 4. Explain how buffers work Copyright © 2008 Pearson Education, Inc. , publishing as Pearson Benjamin Cummings
 Chapter 3 water and the fitness of the environment
Chapter 3 water and the fitness of the environment Water and water and water water
Water and water and water water Skills related
Skills related Environment of business finance
Environment of business finance Class 8 english chapter 7 water water everywhere
Class 8 english chapter 7 water water everywhere Lifelong fitness formula
Lifelong fitness formula Chapter 7 nutrition and your fitness
Chapter 7 nutrition and your fitness Chapter 7 nutrition and your fitness
Chapter 7 nutrition and your fitness Chapter 4 nutrition and your personal fitness
Chapter 4 nutrition and your personal fitness Chapter 12 lesson 1 benefits of physical activity
Chapter 12 lesson 1 benefits of physical activity Chapter 1 fitness and wellness for all answers
Chapter 1 fitness and wellness for all answers Chapter 12 lesson 3 planning a personal activity program
Chapter 12 lesson 3 planning a personal activity program Seven fitness nutrition
Seven fitness nutrition Chapter 12 lesson 2 improving your fitness
Chapter 12 lesson 2 improving your fitness Chapter 1 fitness and wellness for all answers
Chapter 1 fitness and wellness for all answers Ciwem membership
Ciwem membership Iowa water environment association
Iowa water environment association Rocky mountain water environment association
Rocky mountain water environment association Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân