Chapter 3 QUANTITY OF HEAT A M NASR












- Slides: 22

Chapter (3) QUANTITY OF HEAT A. M. NASR

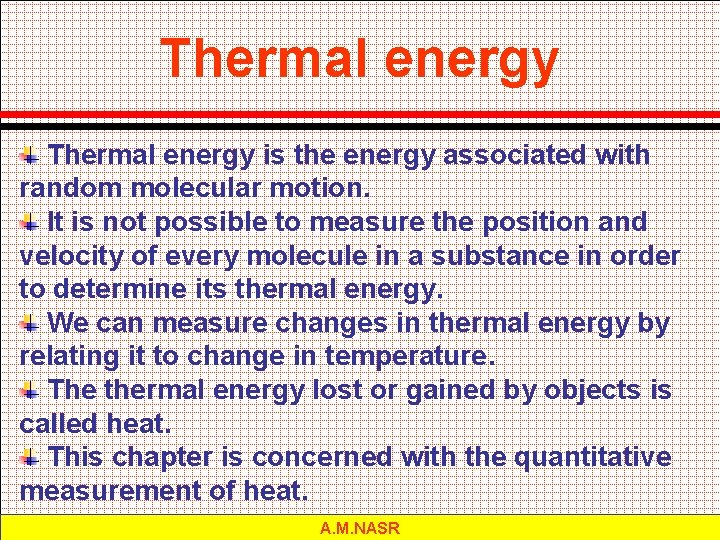
Thermal energy is the energy associated with random molecular motion. It is not possible to measure the position and velocity of every molecule in a substance in order to determine its thermal energy. We can measure changes in thermal energy by relating it to change in temperature. The thermal energy lost or gained by objects is called heat. This chapter is concerned with the quantitative measurement of heat. A. M. NASR

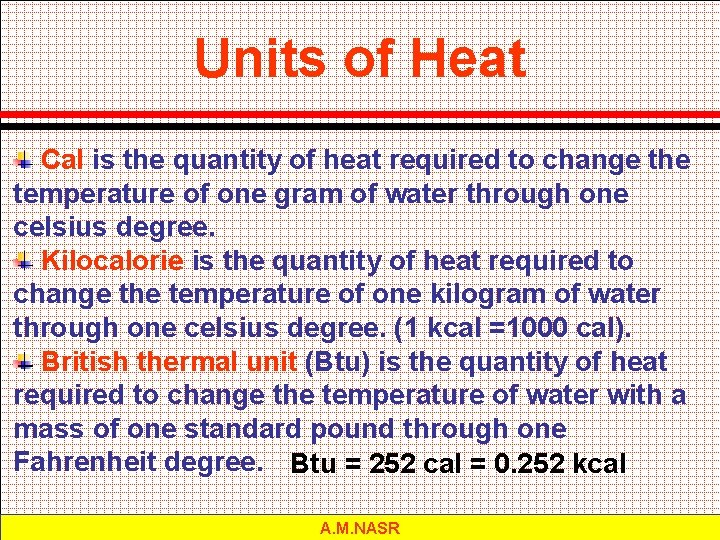
Units of Heat Cal is the quantity of heat required to change the temperature of one gram of water through one celsius degree. Kilocalorie is the quantity of heat required to change the temperature of one kilogram of water through one celsius degree. (1 kcal =1000 cal). British thermal unit (Btu) is the quantity of heat required to change the temperature of water with a mass of one standard pound through one Fahrenheit degree. Btu = 252 cal = 0. 252 kcal A. M. NASR

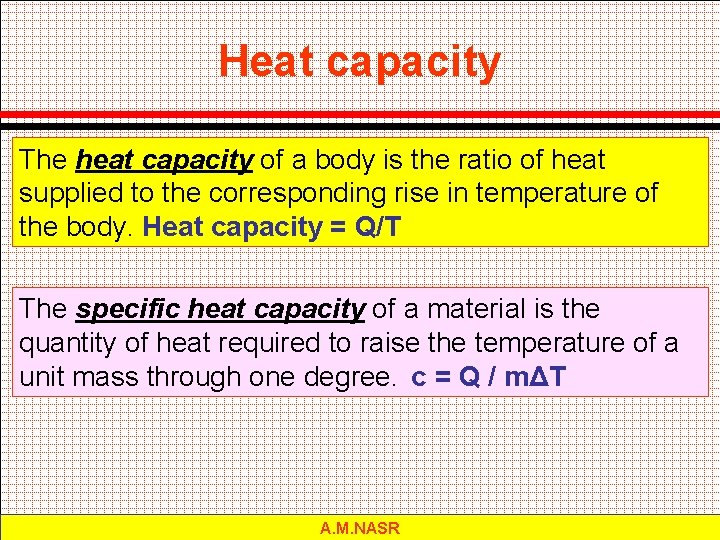
Heat capacity The heat capacity of a body is the ratio of heat supplied to the corresponding rise in temperature of the body. Heat capacity = Q/T The specific heat capacity of a material is the quantity of heat required to raise the temperature of a unit mass through one degree. c = Q / mΔT A. M. NASR

Example 3. 1 How much heat is required to raise the temperature of 200 g of mercury from 20 to 100 K? ) c= 0. 033 cal/go. C) Q = mcΔT = (200 g)(0. 033 cal/go. C)(100 o. C -20 o. C) = 528 cal A. M. NASR

Example 3. 2 During a bout with the flu an 80 -kg man ran a fever of 2 o. C above normal; that is, his body temperature was 39 o. C instead of the normal 37 o. C. Assuming that the human body is mostly water (c=1 kcal/kgo. C), how much heat is required to raise his temperature by that amount? A. M. NASR

Example 3. 2 A. M. NASR

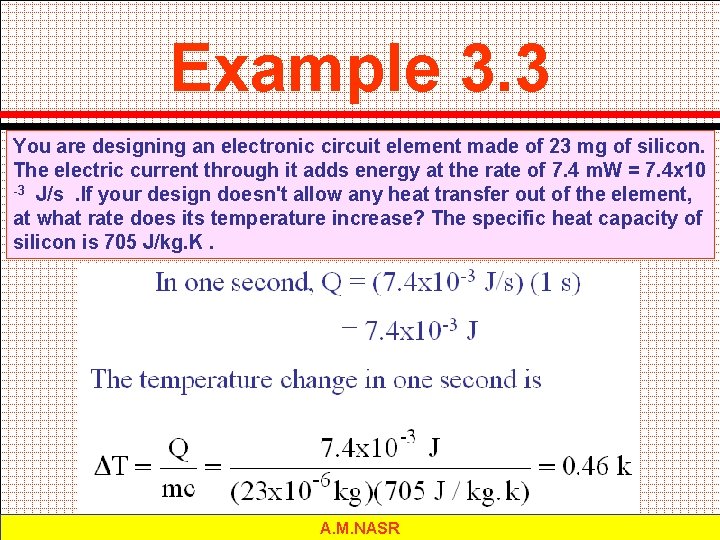
Example 3. 3 You are designing an electronic circuit element made of 23 mg of silicon. The electric current through it adds energy at the rate of 7. 4 m. W = 7. 4 x 10 -3 J/s. If your design doesn't allow any heat transfer out of the element, at what rate does its temperature increase? The specific heat capacity of silicon is 705 J/kg. K. A. M. NASR

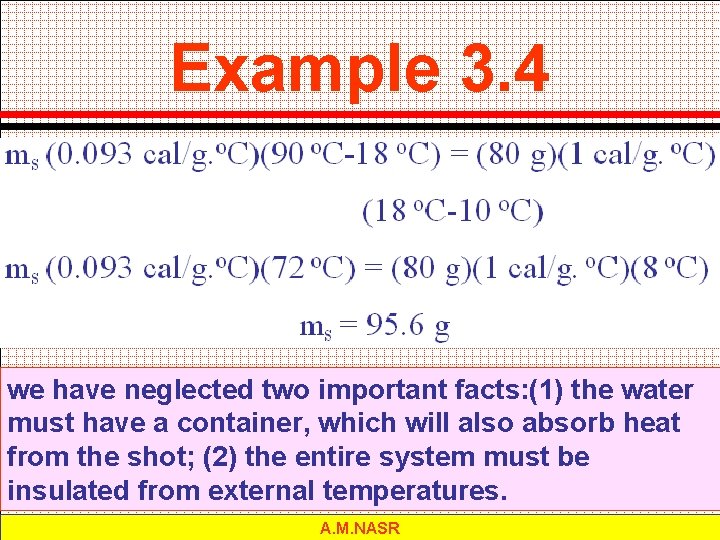
Example 3. 4 A handful of copper shot is heated to 90 o. C and then dropped into 80 g of water at 10 o. C. The final temperature of the mixture is 18 o. C. What was the mass of the shot? A. M. NASR

Example 3. 4 we have neglected two important facts: (1) the water must have a container, which will also absorb heat from the shot; (2) the entire system must be insulated from external temperatures. A. M. NASR

Example 3. 5 In a laboratory experiment it is desired to use a calorimeter to find the specific heat of iron. Eighty grams of dry iron shot is placed in a cup and heated to temperature of 95 o. C. The mass of the inner aluminum cup and of the aluminum stirrer is 60 g. The calorimeter is partially filled with 150 g of water at 18 o. C. The hot shot is quickly poured into the cup, and the calorimeter is sealed. After the system has reached thermal equilibrium, the final temperature is 22 o. C. Compute the specific heat of iron. A. M. NASR

Example 3. 5 Qw =mc T=(150 g)(1 cal/go. C)(22 o. C -18 o. C)= 600 cal Qal = mc T = (60 g)(0. 22 cal/go. C)( 22 o. C -18 o. C) = 52. 8 cal Heat gained = 600 cal + 52. 8 cal = 652. 8 cal The heat lost by the iron shot: = mcs ΔT = (80 g)cs (95 C-22 o. C) The heat lost equal to the heat gained (80 g)cs(73 o. C) = 652. 8 cal Cs = A. M. NASR

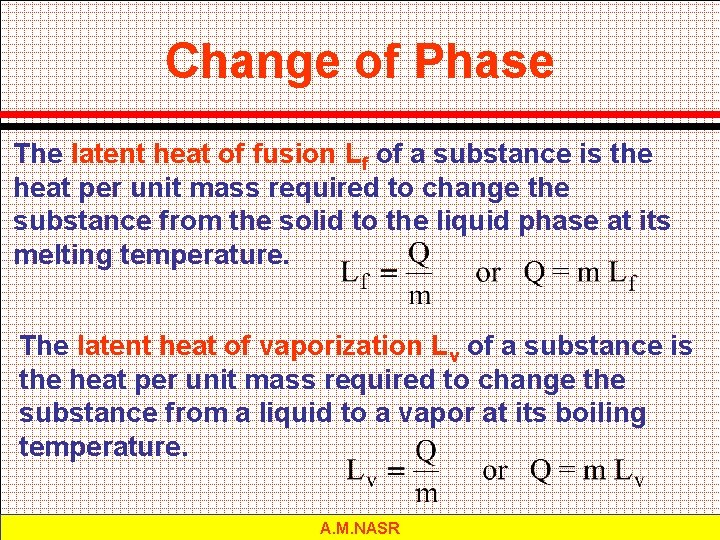
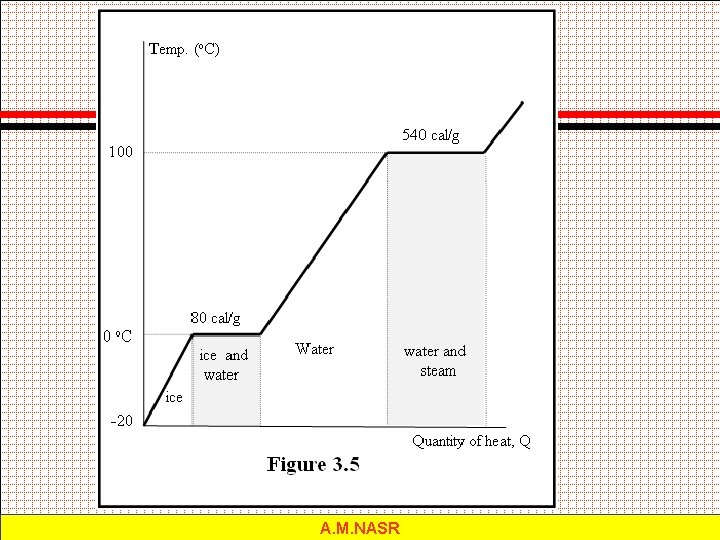
Change of Phase The latent heat of fusion Lf of a substance is the heat per unit mass required to change the substance from the solid to the liquid phase at its melting temperature. The latent heat of vaporization Lv of a substance is the heat per unit mass required to change the substance from a liquid to a vapor at its boiling temperature. A. M. NASR

A. M. NASR

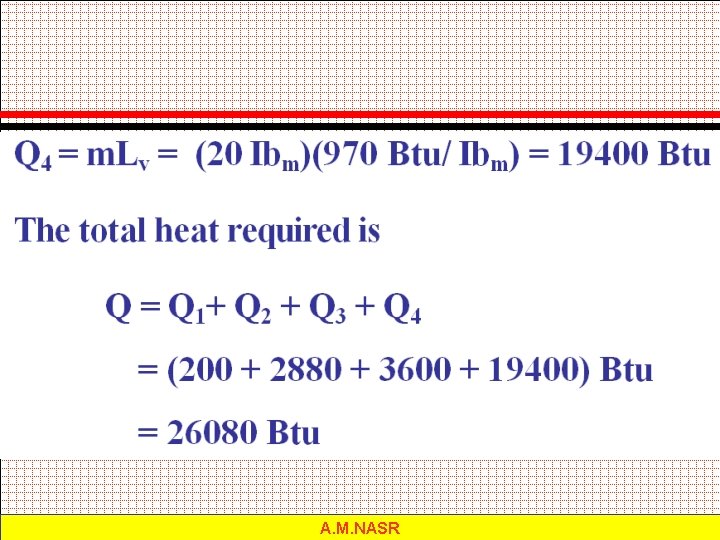
Example 3. 6: What quantity of heat is required to change 20 lbm of ice at 12 F to steam at 212 F? A. M. NASR

Example 3. 6: What quantity of heat is required to change 20 lbm of ice at 12 F to steam at 212 F? A. M. NASR

A. M. NASR

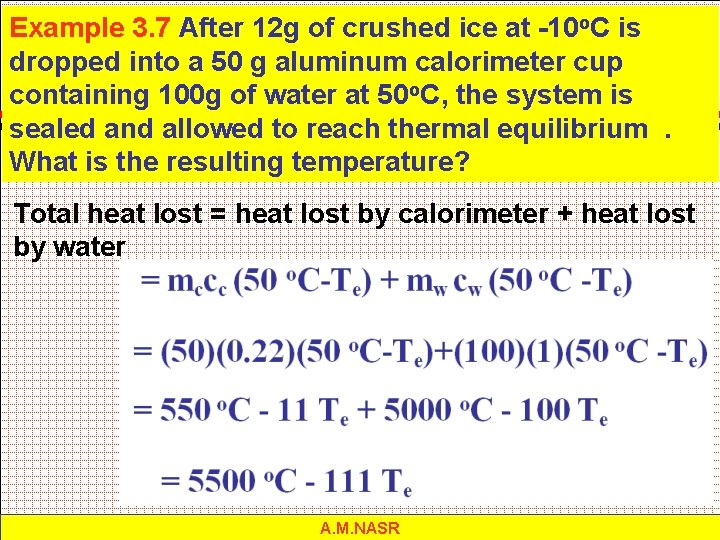
Example 3. 7 After 12 g of crushed ice at -10 o. C is dropped into a 50 g aluminum calorimeter cup containing 100 g of water at 50 o. C, the system is sealed and allowed to reach thermal equilibrium. What is the resulting temperature? Total heat lost = heat lost by calorimeter + heat lost by water A. M. NASR

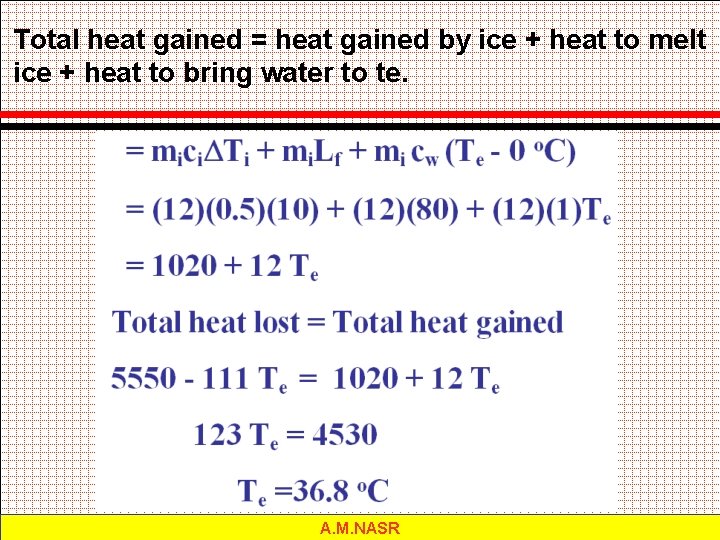
Total heat gained = heat gained by ice + heat to melt ice + heat to bring water to te. A. M. NASR

Example 3. 8 If 10 g of steam at 100 C is introduced into a mixture of 200 g of water and 120 g of ice. find the final temperature and composition of the mixture. The heat required to melt the 120 g of ice completely at 0 o. C. Q 1 = mi Lf = (120 g)(80 cal/g) = 9600 cal The maximum heat we can expect the steam to give up is Q 2 = ms Lv + ms cw (100 o. C - 0 o. C) = (10)(540) + (10)(1)(100) = 6400 cal To determine the final composition of the mixture, note that 3200 additional calories would be required to melt the remaining ice. Hence A. M. NASR

A. M. NASR

 Is mass a scalar quantity
Is mass a scalar quantity Scalar and vector quantities
Scalar and vector quantities Is magnitude scalar or vector
Is magnitude scalar or vector Y varies inversely as x example
Y varies inversely as x example Scalar quantity
Scalar quantity Abu nasr farobiyning asarlaridagi pedagogik fikrlar
Abu nasr farobiyning asarlaridagi pedagogik fikrlar Asr suresi ve anlamı okunuşu
Asr suresi ve anlamı okunuşu Dr reza nasr
Dr reza nasr Mnemonic for mechanism of labour
Mnemonic for mechanism of labour Reza nasr
Reza nasr Bockhart impetigo treatment
Bockhart impetigo treatment Dr samer nasr
Dr samer nasr Nars suresi
Nars suresi Faa nasr
Faa nasr Dr reza nasr
Dr reza nasr Ramzi nasr
Ramzi nasr Apakah pokok-pokok isi surat an-nasr
Apakah pokok-pokok isi surat an-nasr Dr mohamed nasr
Dr mohamed nasr Kamal nasr
Kamal nasr Sam nasr
Sam nasr Specific heat of water
Specific heat of water Latent heat problems
Latent heat problems Combination cooking methods
Combination cooking methods