Chapter 2 Measurements and Calculations Units of Measurement







- Slides: 25

Chapter 2: Measurements and Calculations

Units of Measurement • A standard system for measurements called Le Systeme Internatitional d’Unites was adopted by the General Conference on Weights and Measures in 1960. • These units of measurements are abbreviated SI units.

Units of Measurement Table 2 -1 on page 34 of your text lists the 7 base SI units. Quantity Unit Name Length meter Mass kilogram Time second Temperature Kelvin Amount of Mole Substance Abbreviation m kg s K mol

SI Prefixes (P 35) Prefix- Symbol Exponent Factor Value Tera T 1012 1 000 000 Giga G 109 1 000 000 Mega M 106 1 000 Kilo k 103 1000 Hecto h 102 100 Deka da 101 10 Deci d 10 -1 0. 1 Centi c 10 --2 0. 01 Milli m 10 -3 0. 001 Micro μ 10 -6 0. 000 001 10 -9 0. 000 001 Nano n

Units of Measurement Page 36 • The base SI units may be combined to form derived units. • For example volume has three dimensions. Therefore volume can be expressed as m x m = m 3 • Table 2 -2 on page 35 of your text lists the SI prefixes. Please review these.

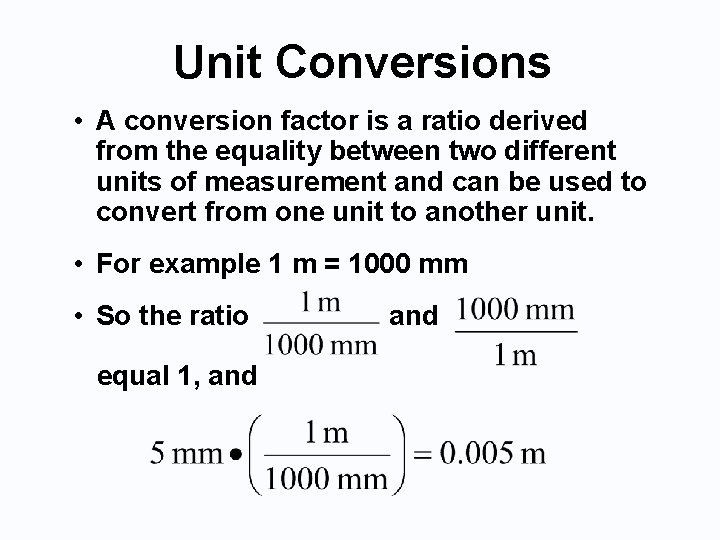
Unit Conversions • A conversion factor is a ratio derived from the equality between two different units of measurement and can be used to convert from one unit to another unit. • For example 1 m = 1000 mm • So the ratio equal 1, and

Unit Conversions Your Turn: If you have 350 mg, how many grams do you have? Table 2 -2 on page 35 of your text lists the SI prefixes.

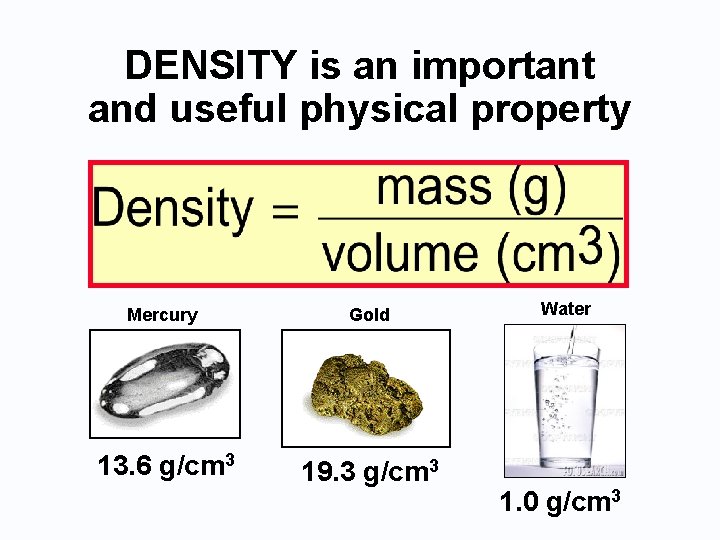
DENSITY is an important and useful physical property Mercury Gold 13. 6 g/cm 3 19. 3 g/cm 3 Water 1. 0 g/cm 3

Water • Water is used as one of the bases for the SI unit system. • The density of water is set to 1. 0 g/cm 3, in other words, 1 cm 3 of water weighs 1 g. Also 1 m. L is equivalent to 1 cm 3. • Therefore 1 g = 1 cm 3 = 1 m. L

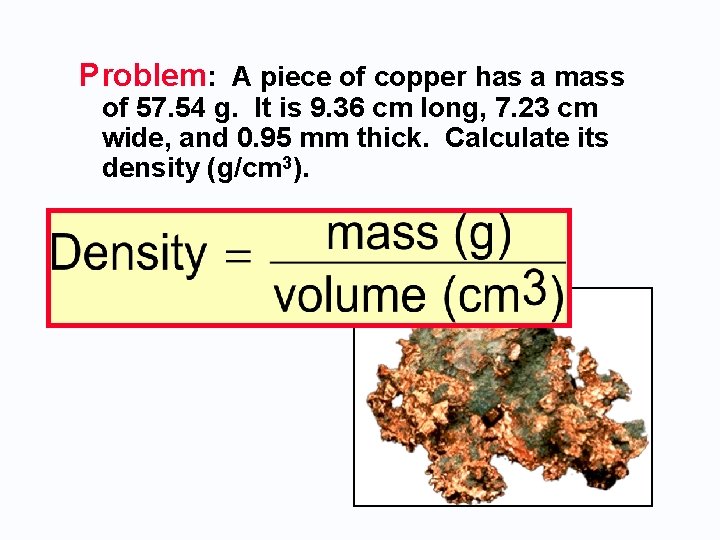
Problem: A piece of copper has a mass of 57. 54 g. It is 9. 36 cm long, 7. 23 cm wide, and 0. 95 mm thick. Calculate its density (g/cm 3).

SOLUTION 1. Get dimensions in common units. 0. 95 mm • 1 cm = 0. 095 cm 10 mm

SOLUTION 1. Get dimensions in common units. 0. 95 mm • 1 cm = 0. 095 cm 10 mm 2. Calculate volume in cubic centimeters. (9. 36 cm)(7. 23 cm)(0. 095 cm) = 6. 4 cm 3 3. Calculate the density. 57. 54 g = 9. 0 g/cm 3 6. 4 cm 3

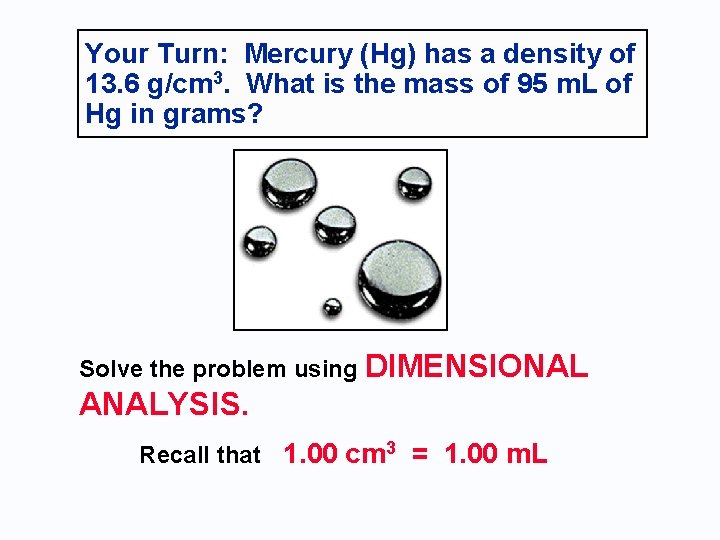
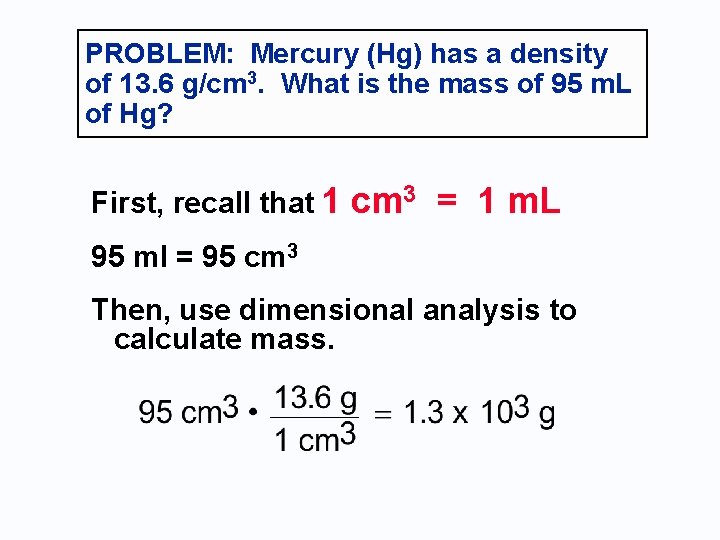
Your Turn: Mercury (Hg) has a density of 13. 6 g/cm 3. What is the mass of 95 m. L of Hg in grams? Solve the problem using DIMENSIONAL ANALYSIS. Recall that 1. 00 cm 3 = 1. 00 m. L

PROBLEM: Mercury (Hg) has a density of 13. 6 g/cm 3. What is the mass of 95 m. L of Hg? First, recall that 1 cm 3 = 1 m. L 95 ml = 95 cm 3 Then, use dimensional analysis to calculate mass.

Practice • What is the volume of a sample of liquid mercury that has a mass of 76. 2 g given that the density of mercury is 13. 6 g/m. L?

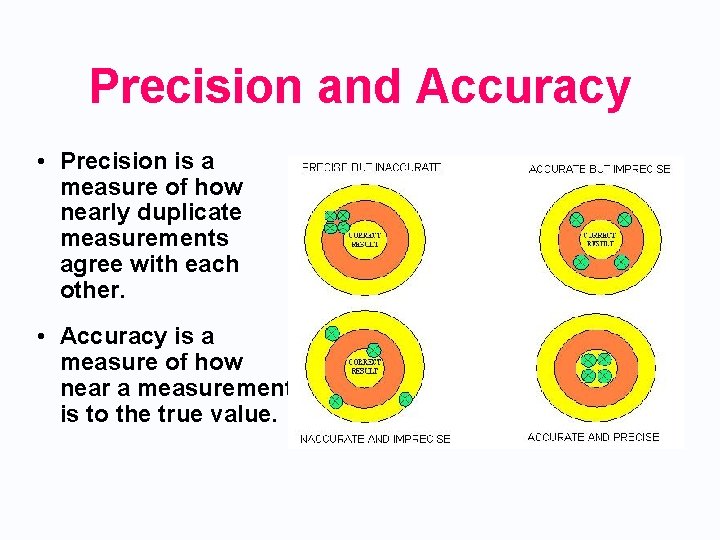
Precision and Accuracy • Precision is a measure of how nearly duplicate measurements agree with each other. • Accuracy is a measure of how near a measurement is to the true value.

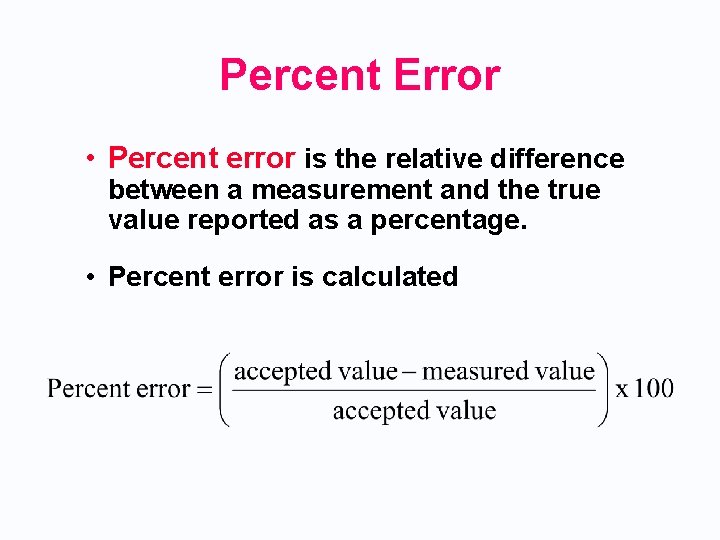
Percent Error • Percent error is the relative difference between a measurement and the true value reported as a percentage. • Percent error is calculated

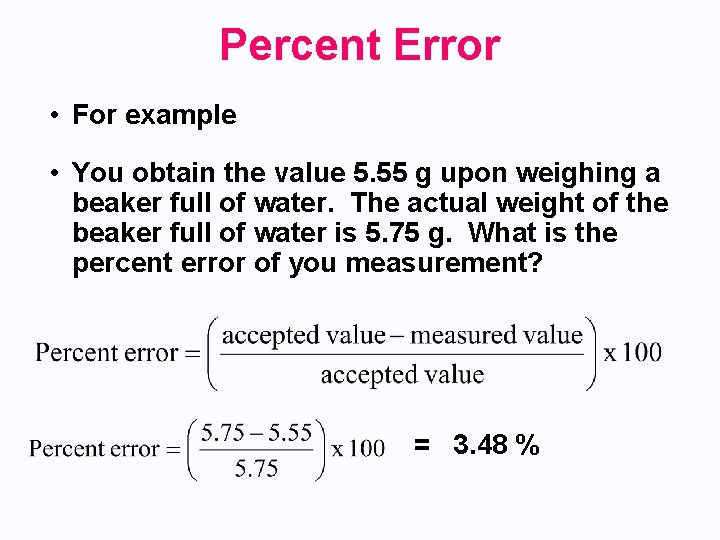
Percent Error • For example • You obtain the value 5. 55 g upon weighing a beaker full of water. The actual weight of the beaker full of water is 5. 75 g. What is the percent error of you measurement? = 3. 48 %

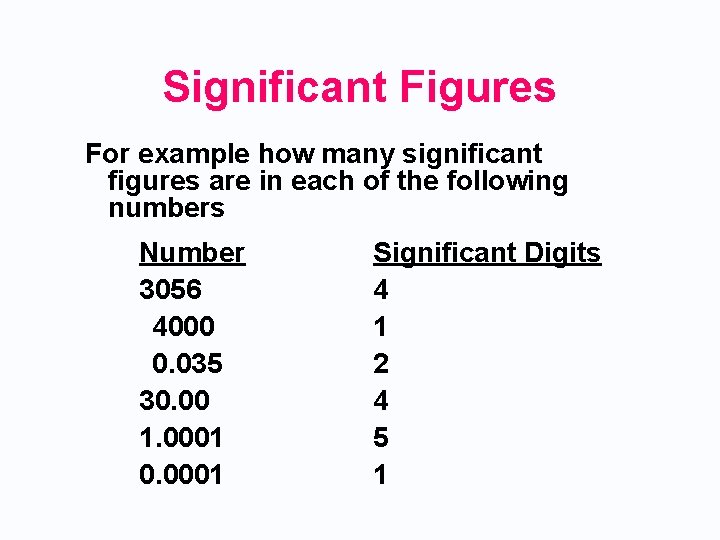
Significant Figures • All nonzero digits are significant. • All zeros that lie between nonzero digits are significant. • None of the zeros that lie to the left, or in front, of the first nonzero digit are significant. • Zeros to the right of the last nonzero digit are significant ONLY if they lie to the right of the decimal point.

Significant Figures For example how many significant figures are in each of the following numbers Number 3056 4000 0. 035 30. 00 1. 0001 0. 0001 Significant Digits 4 1 2 4 5 1

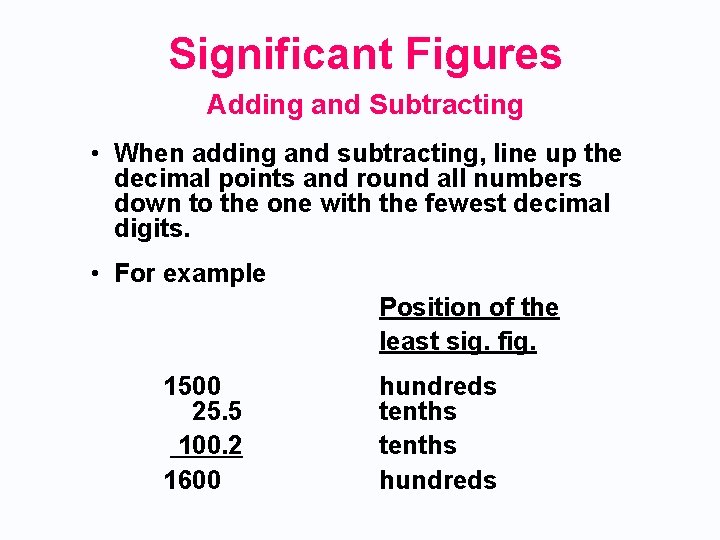
Significant Figures Adding and Subtracting • When adding and subtracting, line up the decimal points and round all numbers down to the one with the fewest decimal digits. • For example Position of the least sig. fig. 1500 25. 5 100. 2 1600 hundreds tenths hundreds

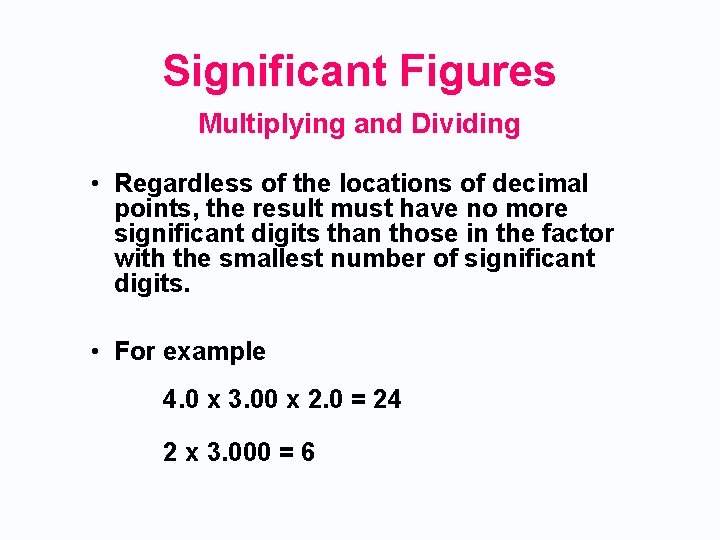
Significant Figures Multiplying and Dividing • Regardless of the locations of decimal points, the result must have no more significant digits than those in the factor with the smallest number of significant digits. • For example 4. 0 x 3. 00 x 2. 0 = 24 2 x 3. 000 = 6

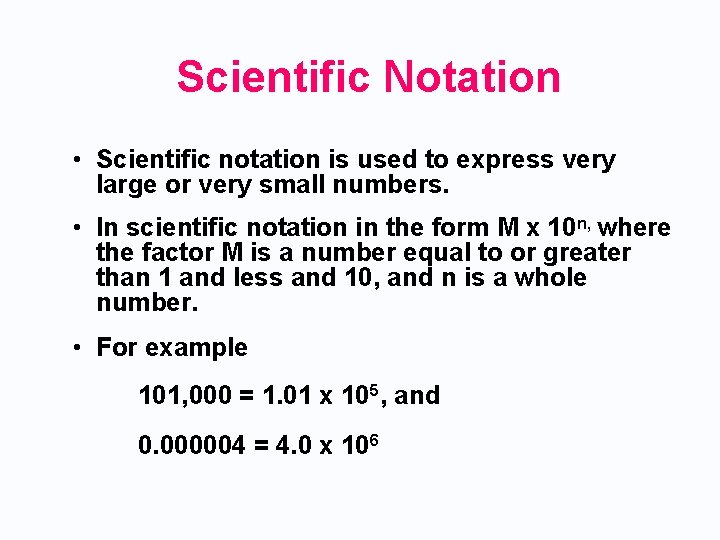
Scientific Notation • Scientific notation is used to express very large or very small numbers. • In scientific notation in the form M x 10 n, where the factor M is a number equal to or greater than 1 and less and 10, and n is a whole number. • For example 101, 000 = 1. 01 x 105, and 0. 000004 = 4. 0 x 106

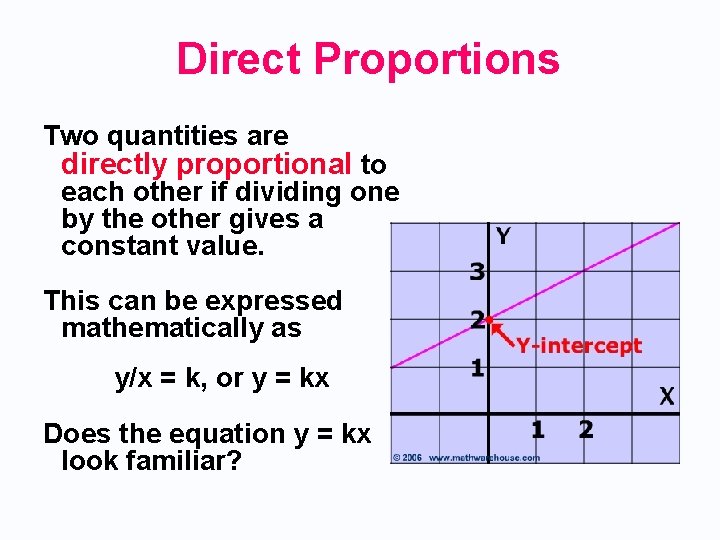
Direct Proportions Two quantities are directly proportional to each other if dividing one by the other gives a constant value. This can be expressed mathematically as y/x = k, or y = kx Does the equation y = kx look familiar?

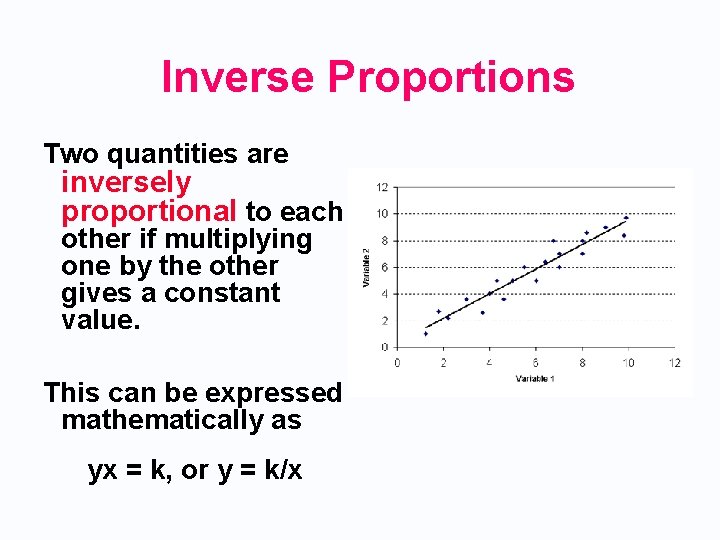
Inverse Proportions Two quantities are inversely proportional to each other if multiplying one by the other gives a constant value. This can be expressed mathematically as yx = k, or y = k/x
 Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Measurements and calculations chapter 2 test
Measurements and calculations chapter 2 test Pharmaceutical measurements and calculations
Pharmaceutical measurements and calculations Pharmacy measurements
Pharmacy measurements Types of connections in steel structures
Types of connections in steel structures Units physical quantities and measurements
Units physical quantities and measurements Units of linear measurement
Units of linear measurement Mussd
Mussd What are the customary units of measurement
What are the customary units of measurement Momentum unit of measurement
Momentum unit of measurement What is customary unit
What is customary unit Typical room height appropriate metric unit
Typical room height appropriate metric unit Us customary units of measurement
Us customary units of measurement Deci centi mili
Deci centi mili Opisometer unit
Opisometer unit Customary and metric system
Customary and metric system Metric system chart
Metric system chart Metric volume units
Metric volume units Metric units
Metric units Metric conversions staircase
Metric conversions staircase Converting metric units
Converting metric units Botox units per ml
Botox units per ml When units manufactured exceed units sold:
When units manufactured exceed units sold: Chapter 37 vital signs and measurements true or false
Chapter 37 vital signs and measurements true or false Vital sign normal
Vital sign normal Gdp types
Gdp types