Measurements SI Units Scientific units that standardize measurements












- Slides: 22

Measurements

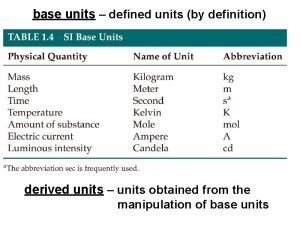
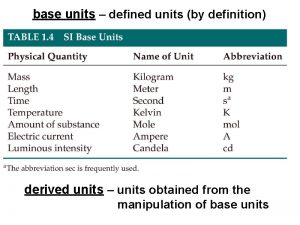
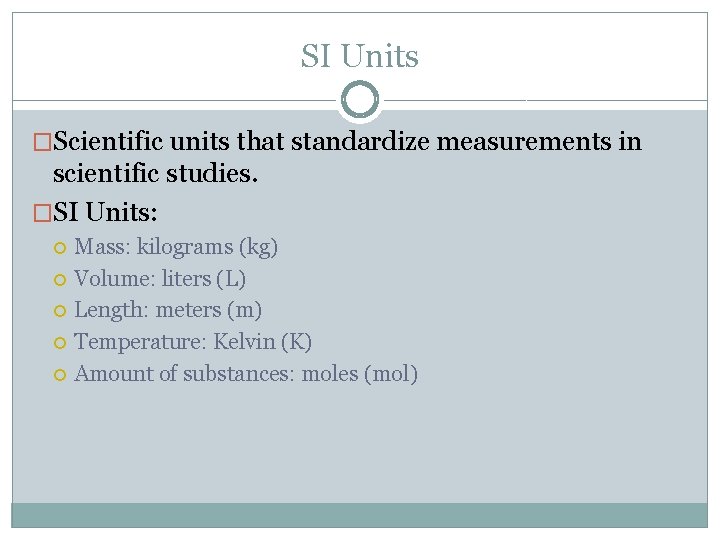
SI Units �Scientific units that standardize measurements in scientific studies. �SI Units: Mass: kilograms (kg) Volume: liters (L) Length: meters (m) Temperature: Kelvin (K) Amount of substances: moles (mol)

Common Units �Units that are more common in experiments. �Common units: Mass: grams (g) Volume: milliliters (m. L) Length: centimeters (cm) Temperature: degrees Celsius (°C) Amount of substance: moles (mol)

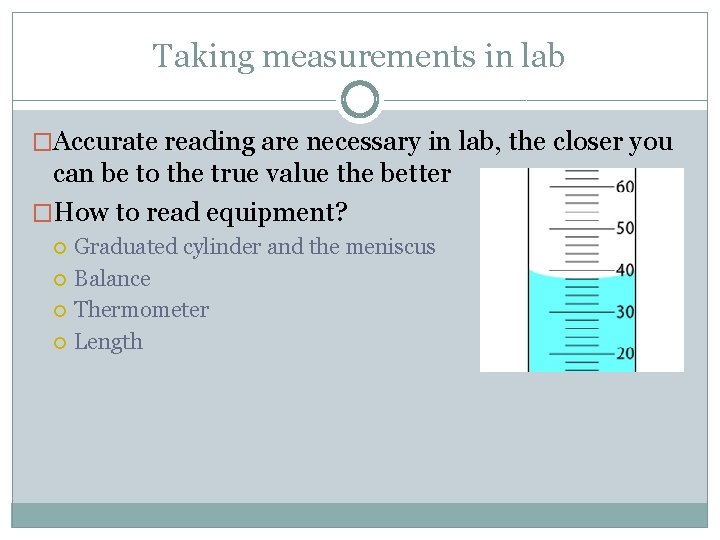
Taking measurements in lab �Accurate reading are necessary in lab, the closer you can be to the true value the better �How to read equipment? Graduated cylinder and the meniscus Balance Thermometer Length

Metric System �Why use it? Universally understood…except 3 countries Makes conversions more simple Prefixes allow for simpler conversions

Scientific Notation �Sometimes measurements are too large or too small to be useful �Change them into a format that makes the data more organized.

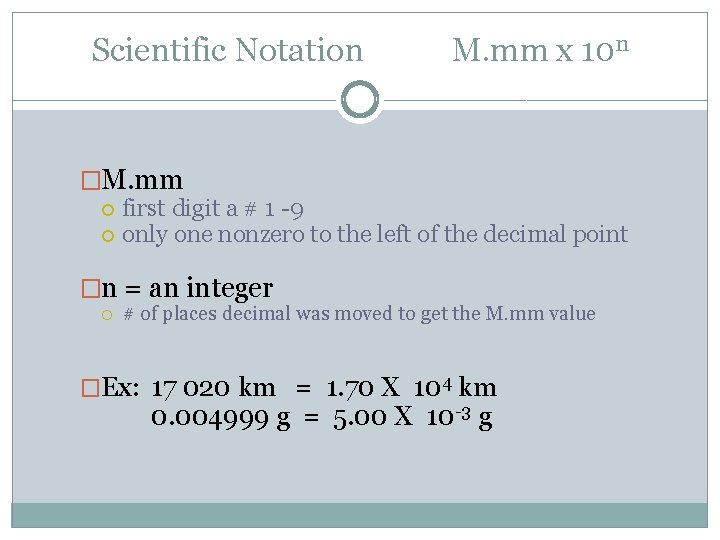
Scientific Notation M. mm x 10 n �M. mm first digit a # 1 -9 only one nonzero to the left of the decimal point �n = an integer # of places decimal was moved to get the M. mm value �Ex: 17 020 km = 1. 70 X 104 km 0. 004999 g = 5. 00 X 10 -3 g

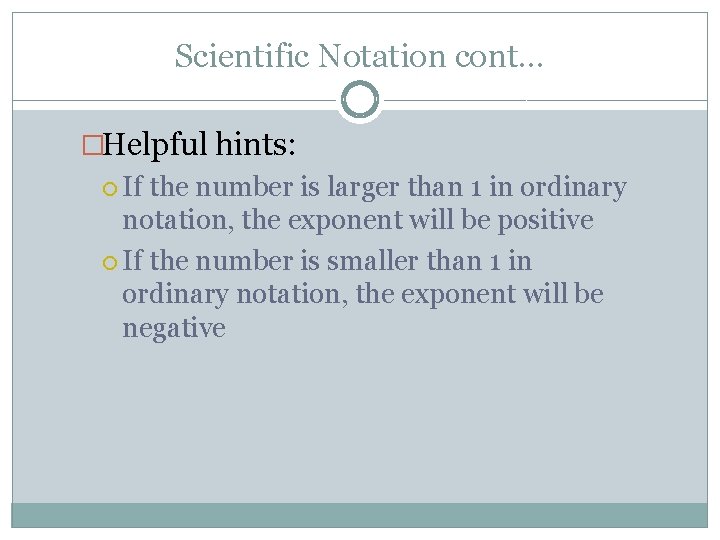
Scientific Notation cont… �Helpful hints: If the number is larger than 1 in ordinary notation, the exponent will be positive If the number is smaller than 1 in ordinary notation, the exponent will be negative

Significant Figures �Used to help in making measurements more precise �Follows a specific set of rules �ALL MATH IN CHEMISTRY MUST USE SIG FIG RULES!!

Significant Figures �Non-zero digits are ALWAYS significant �Ex. 1 L, 2. 3 mm, 456 K �Sandwiched zeros are ALWAYS significant �Ex. 101 m. L, 20103 k. Pa, 2. 00003 g

Significant Figures �Zeros at the end of a number containing a decimal are significant �Ex. 100. g, 0. 0130 L, 20. 0 mg �Zeros in front of a number are NEVER significant �Ex. 0. 00231 m. L, 0. 01 g, 02 atm

Adding/Subtracting �When adding or subtracting, you pay attention to sig figs AFTER the decimal �Examples: 7. 459 km + 82. 3 km – 0. 02 km 1701 g - 50 g + 40 g

Multiplying/Dividing �When multiplying and dividing, you pay attention to ALL SIG FIGS �If doing both addition/subtraction AND multiplication and division, this rule WINS!! �Examples: 651 cm x 75 cm 14. 75 L ÷ 2. 5 L

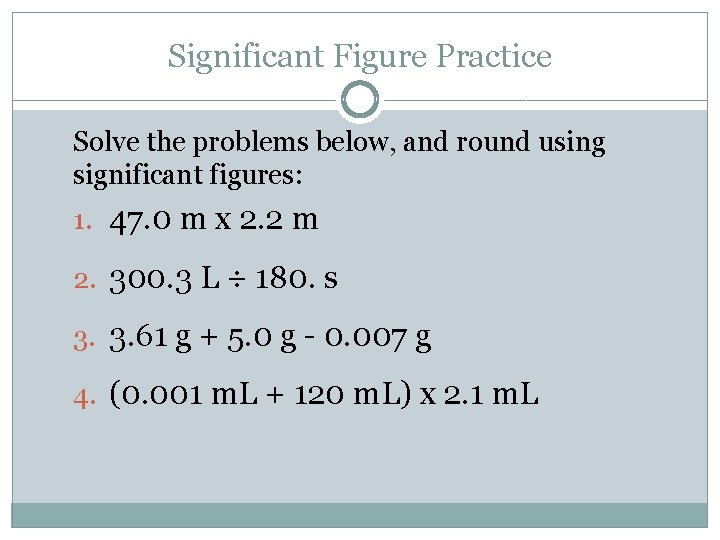
Significant Figure Practice Solve the problems below, and round using significant figures: 1. 47. 0 m x 2. 2 m 2. 300. 3 L ÷ 180. s 3. 3. 61 g + 5. 0 g - 0. 007 g 4. (0. 001 m. L + 120 m. L) x 2. 1 m. L

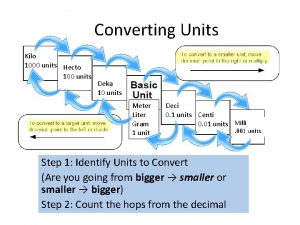
Common Prefixes �Kilo �Hecto �Deka �Deci �Centi �Milli k h dk d c m 103 102 101 10 -2 10 -3

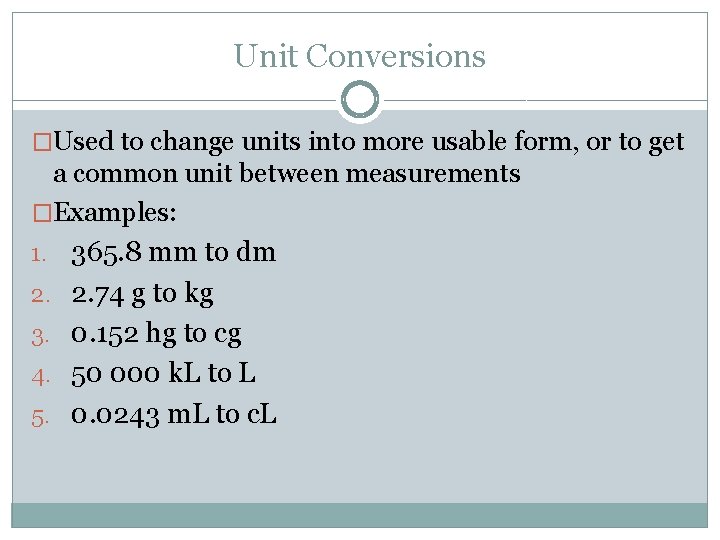
Unit Conversions �Used to change units into more usable form, or to get a common unit between measurements �Examples: 1. 365. 8 mm to dm 2. 2. 74 g to kg 3. 0. 152 hg to cg 4. 50 000 k. L to L 5. 0. 0243 m. L to c. L

Dimensional Analysis �Read the question. �Identify what you want to find. �Identify what you know. �Set up a conversion bridge to solve the problem, using the correct conversion factor.

Dimensional Analysis cont. �Example: In your favorite restaurant, a sandwich you like costs $1. 25. If you order 2 sandwiches, how many quarters must you pay?

Dimensional Analysis (In class practice) 1. Pistachio nuts cost $6. 00 per pound. How many nuts, in grams, can be purchased for $18. 00? (1 lb = 454 g)

Dimensional Analysis (In class practice) 2. How far can Joe drive his car on $30. 00 if the car goes 17. 8 mi/gal and gasoline costs $1. 81 per gal?

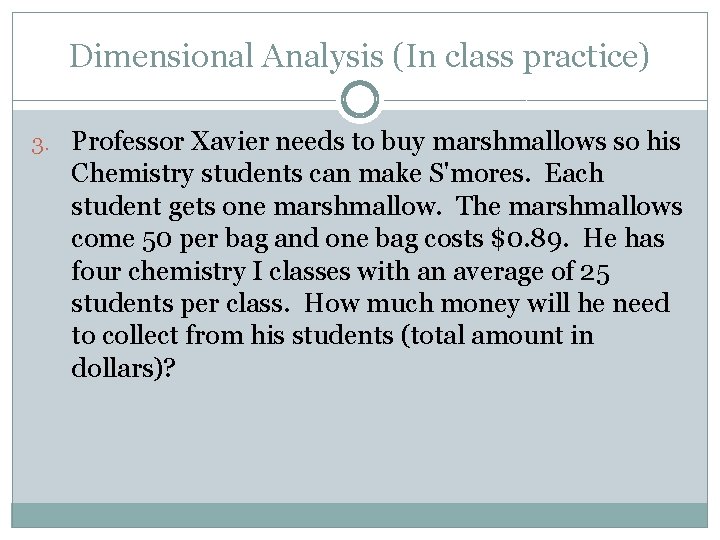
Dimensional Analysis (In class practice) 3. Professor Xavier needs to buy marshmallows so his Chemistry students can make S'mores. Each student gets one marshmallow. The marshmallows come 50 per bag and one bag costs $0. 89. He has four chemistry I classes with an average of 25 students per class. How much money will he need to collect from his students (total amount in dollars)?

Dimensional Analysis (In class practice) �The average speed of the orbiting space shuttle is 17, 500 miles per hour. What is the speed in kilometers per second? (1 mi = 0. 621 km)
 Essa eliminate simplify standardize automate
Essa eliminate simplify standardize automate When all else fails standardize
When all else fails standardize Standardize do check act
Standardize do check act Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Walmart thất bại ở nhật
Walmart thất bại ở nhật Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block xoang nhĩ ecg
Block xoang nhĩ ecg Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thể thơ truyền thống
Thể thơ truyền thống Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Scientific measurements
Scientific measurements Unit 2 lesson 2 scientific notation
Unit 2 lesson 2 scientific notation Derived quantities
Derived quantities 3 units of linear measurements in metric system
3 units of linear measurements in metric system How is a scientific law different from a scientific theory?
How is a scientific law different from a scientific theory? Information gathered during an experiment
Information gathered during an experiment Botox map face
Botox map face Variable costing income statement
Variable costing income statement Instrumentation and measurements
Instrumentation and measurements The two measurements necessary for calculating velocity are
The two measurements necessary for calculating velocity are