Chapter 18 Enols and Enolates Overview Enols Enolates



- Slides: 25

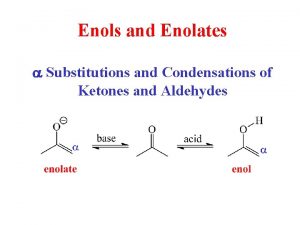
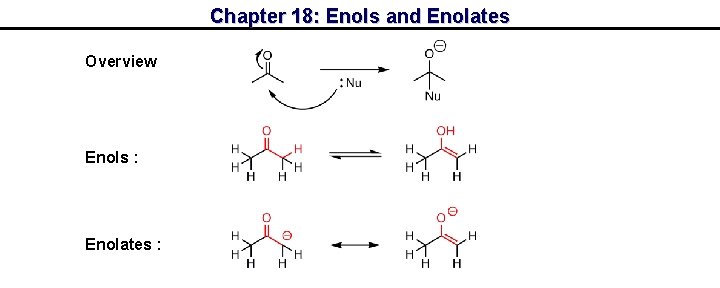
Chapter 18: Enols and Enolates Overview Enols : Enolates :

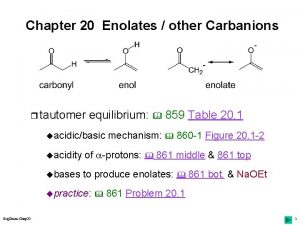
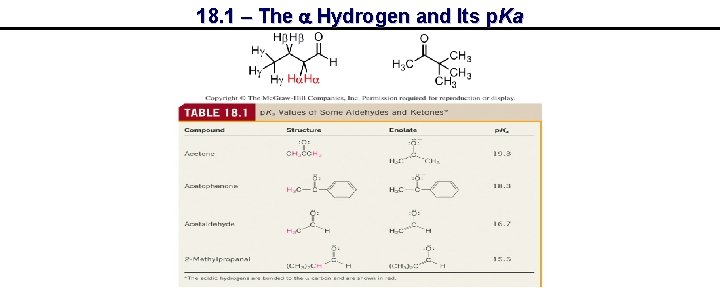
18. 1 – The a Hydrogen and Its p. Ka

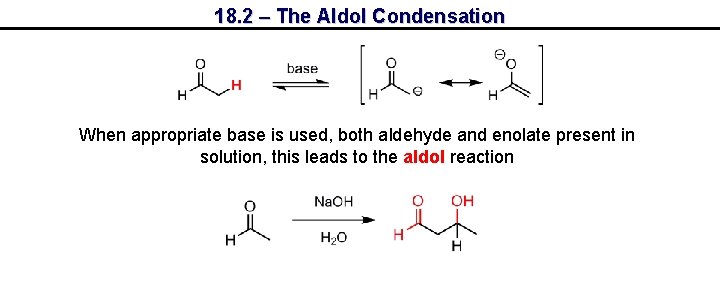
18. 2 – The Aldol Condensation When appropriate base is used, both aldehyde and enolate present in solution, this leads to the aldol reaction

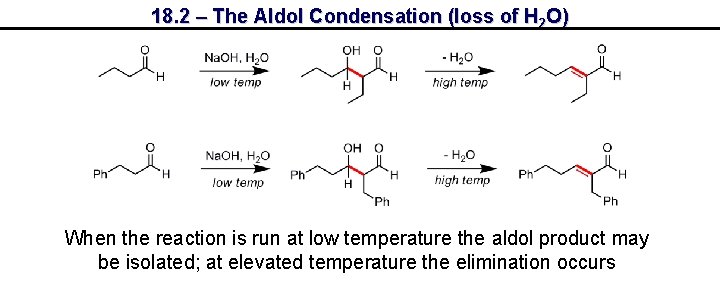
18. 2 – The Aldol Condensation (loss of H 2 O) When the reaction is run at low temperature the aldol product may be isolated; at elevated temperature the elimination occurs

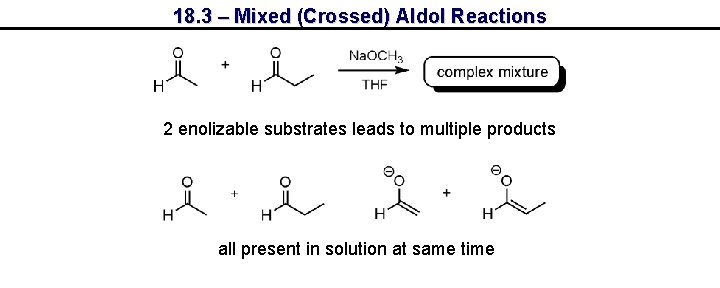
18. 3 – Mixed (Crossed) Aldol Reactions 2 enolizable substrates leads to multiple products all present in solution at same time

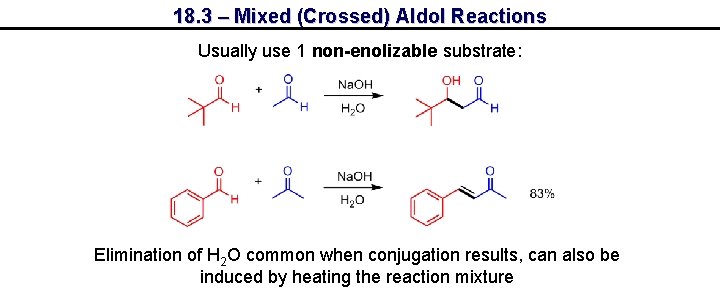
18. 3 – Mixed (Crossed) Aldol Reactions Usually use 1 non-enolizable substrate: Elimination of H 2 O common when conjugation results, can also be induced by heating the reaction mixture

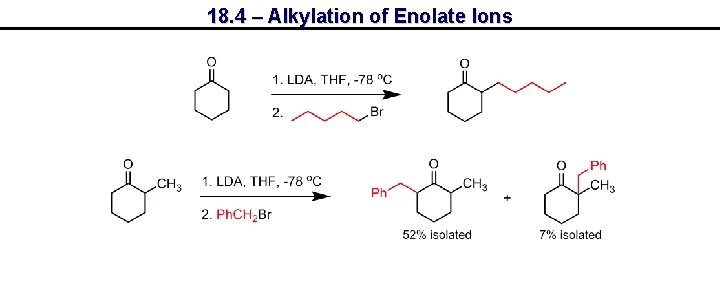
18. 4 – Alkylation of Enolate Ions

Staphylococcus aureus (MRSA, VRSA) • Greek staphyle meaning “a bunch of grapes” • Greek kokkos meaning “berry” • aureus = yellow

• Gram-positive, cluster-forming coccus • Cause food poisoning, endocarditis, osteomyelitis • Can cause septiceamia, infections on implants • Becoming increasingly resistant to antibiotics • MRSA strains appeared in 1961 • VRSA first reported in the USA in 2002

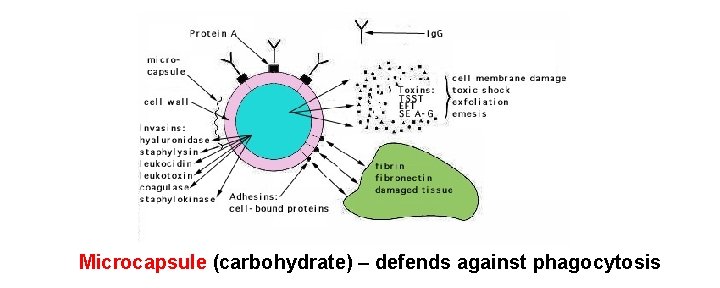
Microcapsule (carbohydrate) – defends against phagocytosis

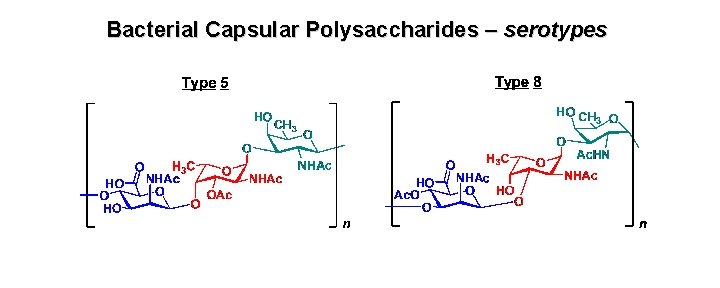
Bacterial Capsular Polysaccharides – serotypes


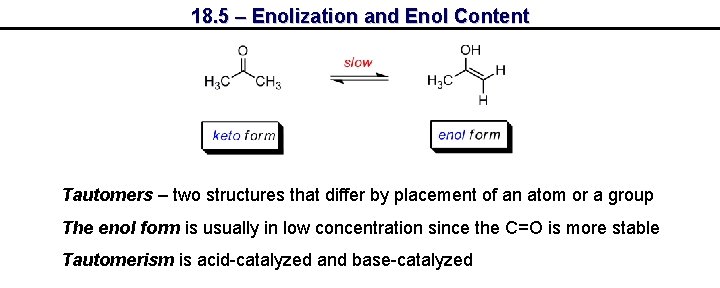
18. 5 – Enolization and Enol Content Tautomers – two structures that differ by placement of an atom or a group The enol form is usually in low concentration since the C=O is more stable Tautomerism is acid-catalyzed and base-catalyzed

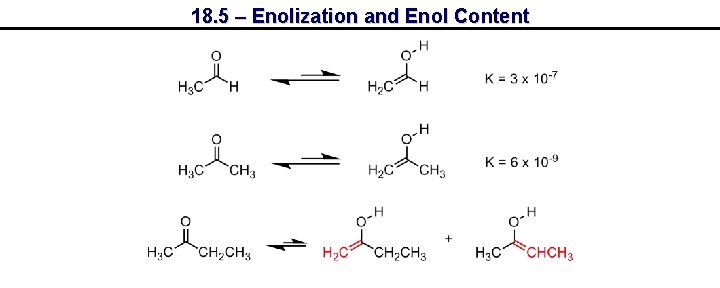
18. 5 – Enolization and Enol Content

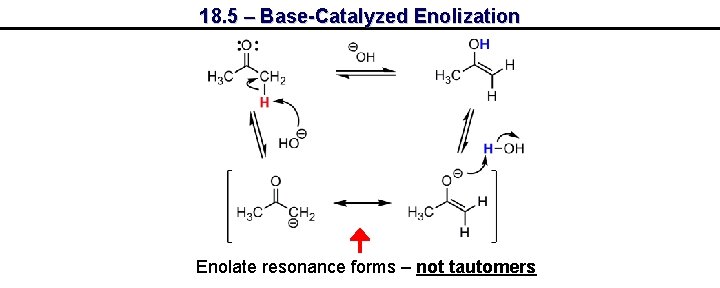
18. 5 – Base-Catalyzed Enolization Enolate resonance forms – not tautomers

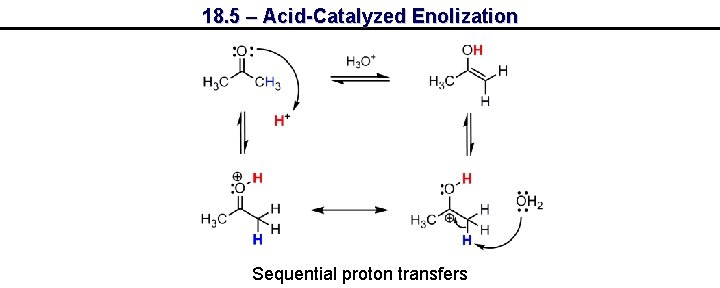
18. 5 – Acid-Catalyzed Enolization Sequential proton transfers

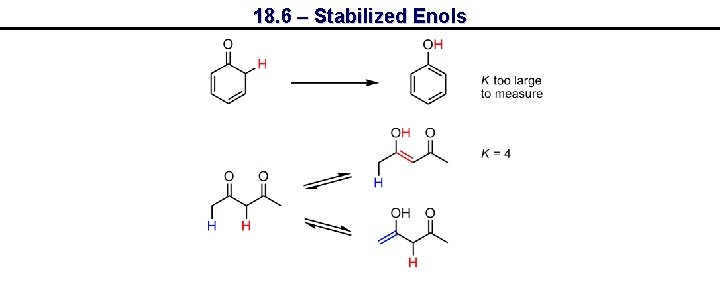
18. 6 – Stabilized Enols

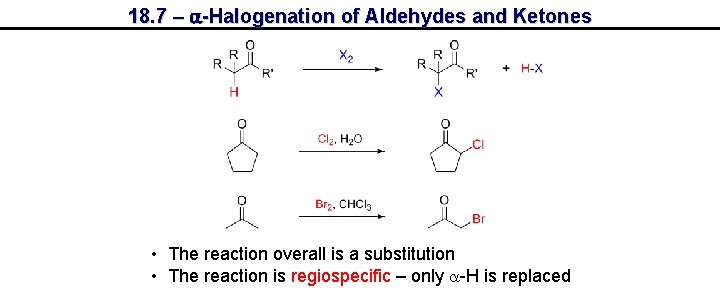
18. 7 – a-Halogenation of Aldehydes and Ketones • The reaction overall is a substitution • The reaction is regiospecific – only a-H is replaced

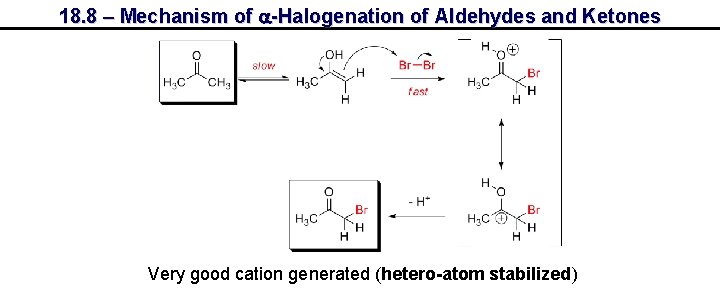
18. 8 – Mechanism of a-Halogenation of Aldehydes and Ketones Very good cation generated (hetero-atom stabilized)

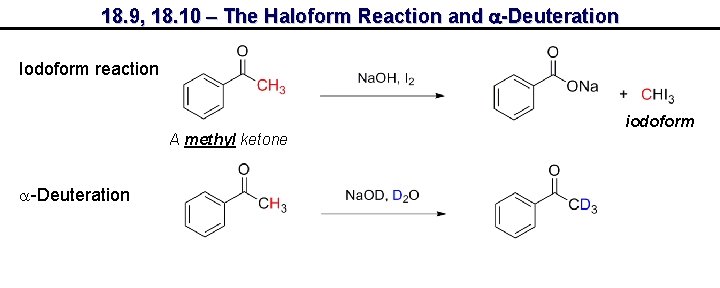
18. 9, 18. 10 – The Haloform Reaction and a-Deuteration Iodoform reaction iodoform A methyl ketone a-Deuteration

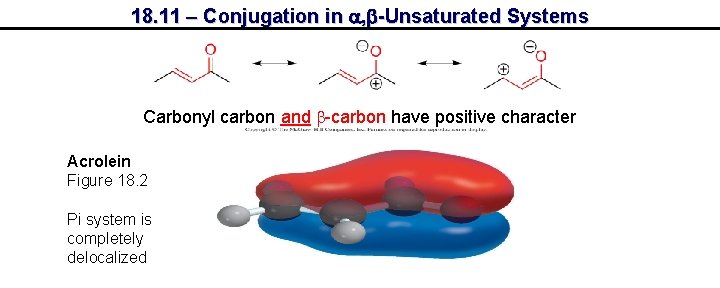
18. 11 – Conjugation in a, b-Unsaturated Systems Carbonyl carbon and b-carbon have positive character Acrolein Figure 18. 2 Pi system is completely delocalized

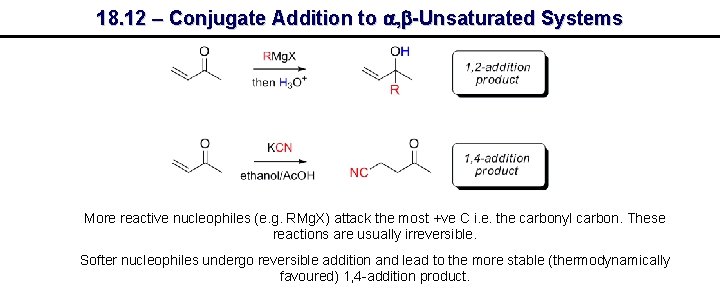
18. 12 – Conjugate Addition to a, b-Unsaturated Systems More reactive nucleophiles (e. g. RMg. X) attack the most +ve C i. e. the carbonyl carbon. These reactions are usually irreversible. Softer nucleophiles undergo reversible addition and lead to the more stable (thermodynamically favoured) 1, 4 -addition product.

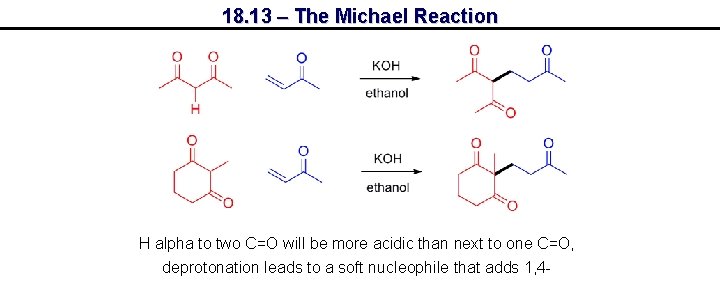
18. 13 – The Michael Reaction H alpha to two C=O will be more acidic than next to one C=O, deprotonation leads to a soft nucleophile that adds 1, 4 -

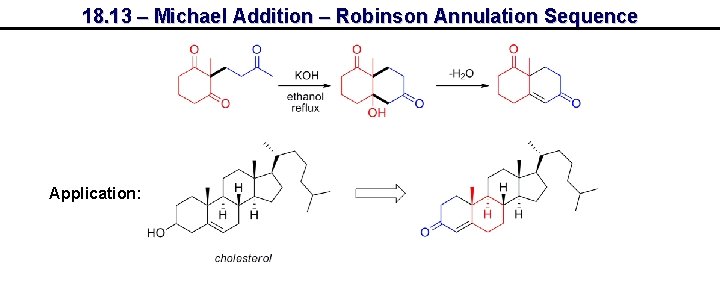
18. 13 – Michael Addition – Robinson Annulation Sequence Application:

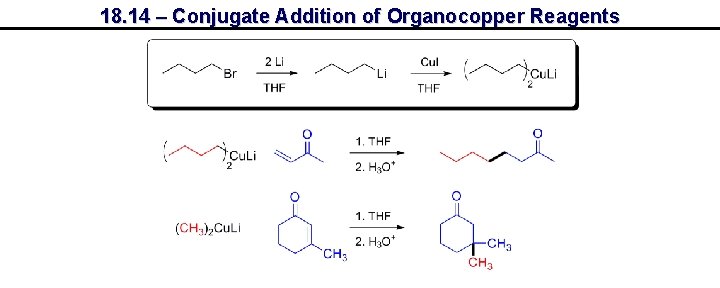
18. 14 – Conjugate Addition of Organocopper Reagents
 Acetoacetic ester synthesis mechanism
Acetoacetic ester synthesis mechanism Enolate
Enolate Elements and their properties section 1 metals
Elements and their properties section 1 metals Chapter 24 trauma overview
Chapter 24 trauma overview Chapter 14 medical overview
Chapter 14 medical overview Chapter 9 lesson 2 photosynthesis an overview
Chapter 9 lesson 2 photosynthesis an overview Chapter 12 selling overview
Chapter 12 selling overview Financial intermediaries
Financial intermediaries Chapter 1 overview of verb tenses
Chapter 1 overview of verb tenses Overview of personal finance chapter 1
Overview of personal finance chapter 1 Distoclusion definition
Distoclusion definition Foundations in personal finance chapter 1
Foundations in personal finance chapter 1 Chapter 32 an overview of animal diversity
Chapter 32 an overview of animal diversity Chapter 1 an overview of financial management
Chapter 1 an overview of financial management Chapter 1 overview of financial statement analysis
Chapter 1 overview of financial statement analysis Perbedaan replikasi virus dna dan rna
Perbedaan replikasi virus dna dan rna Data cleaning problems and current approaches
Data cleaning problems and current approaches Elements and their properties section 1 metals answer key
Elements and their properties section 1 metals answer key Chicago time
Chicago time Multicullar
Multicullar An overview of data warehousing and olap technology
An overview of data warehousing and olap technology Data quality and data cleaning an overview
Data quality and data cleaning an overview Data quality and data cleaning an overview
Data quality and data cleaning an overview Overview of storage and indexing
Overview of storage and indexing Overview of www
Overview of www Maximo overview
Maximo overview