Ch 2 Water Air and Their Impurities 2











- Slides: 37

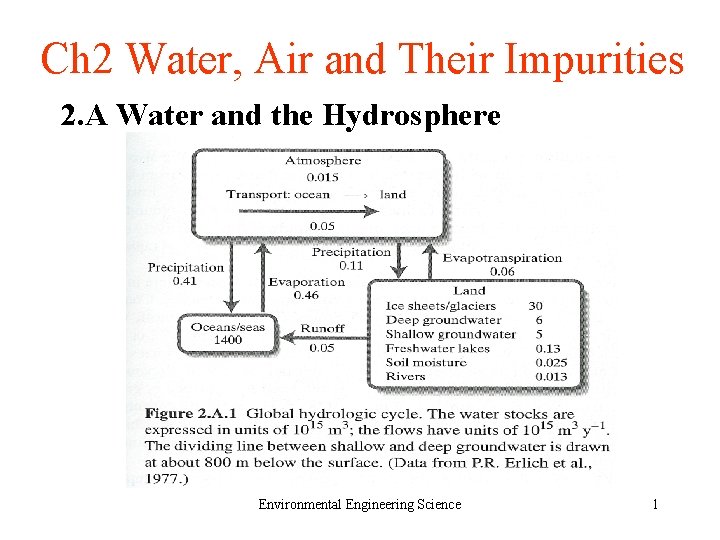
Ch 2 Water, Air and Their Impurities 2. A Water and the Hydrosphere Environmental Engineering Science 1


Water and hydrosphere • Hydrologic cycle – Characteristic residence times • Physicochemical properties – Density (1 g/cm 3, 55. 6 M, Temp. , Composition) – Density of ice 0. 92 g/cm 3 – Viscosity (0. 6 -1. 8 g/m. s in 0 -40 o. C) – Water and Life • O 2 → H 2 O Environmental Engineering Science 3


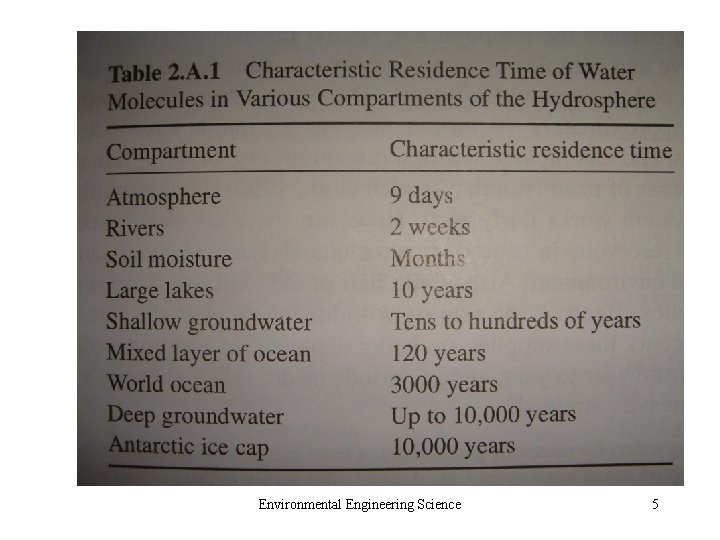
Environmental Engineering Science 5

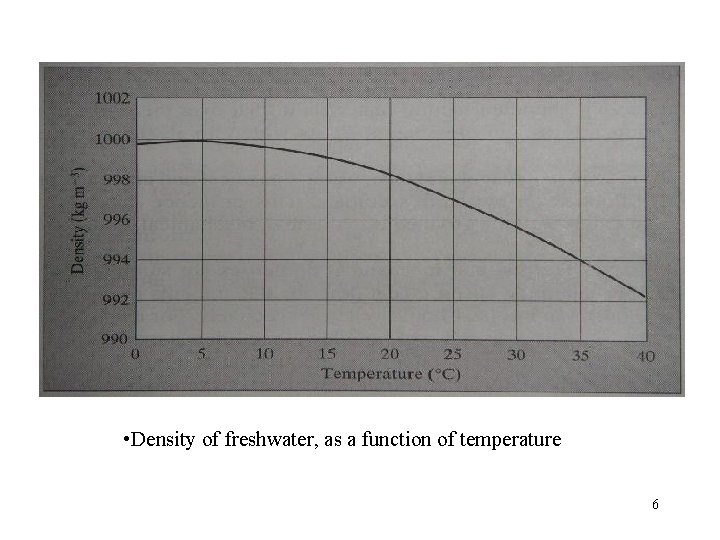
• Density of freshwater, as a function of temperature 6

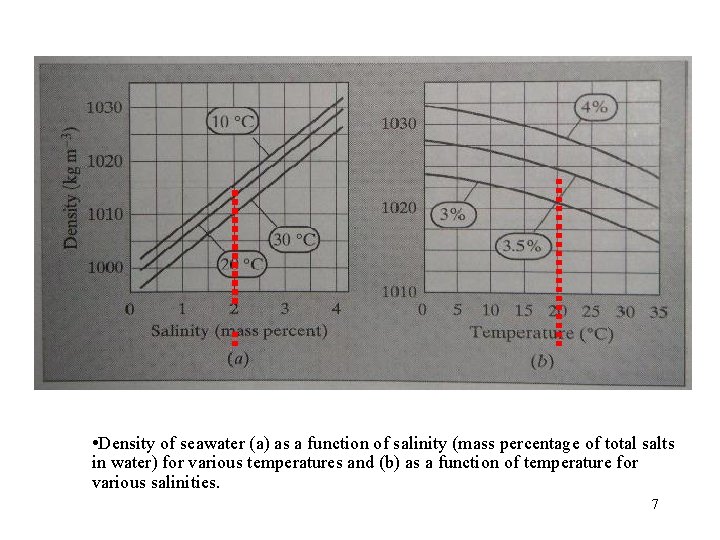
• Density of seawater (a) as a function of salinity (mass percentage of total salts in water) for various temperatures and (b) as a function of temperature for various salinities. 7

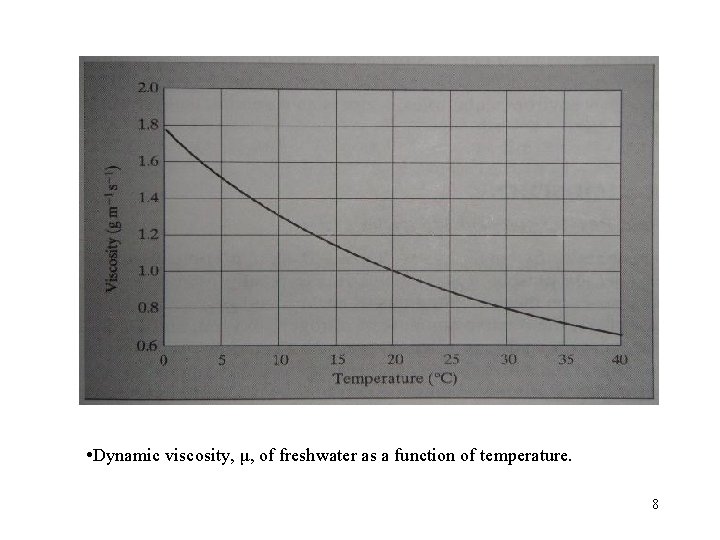
• Dynamic viscosity, μ, of freshwater as a function of temperature. 8

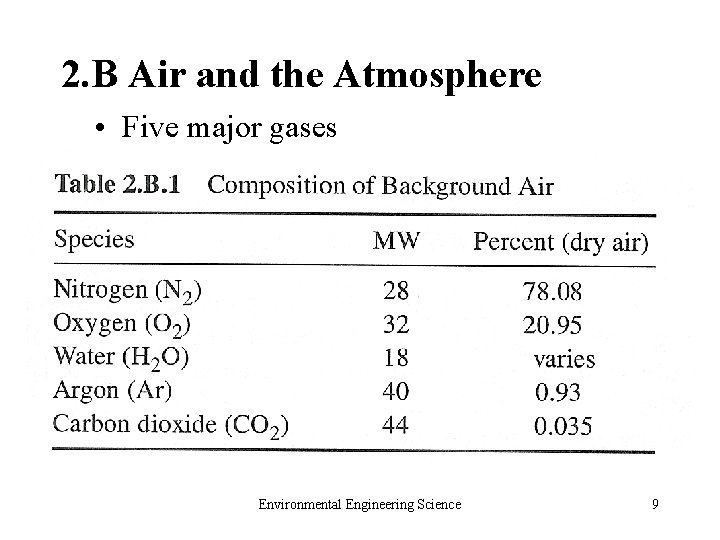
2. B Air and the Atmosphere • Five major gases Environmental Engineering Science 9

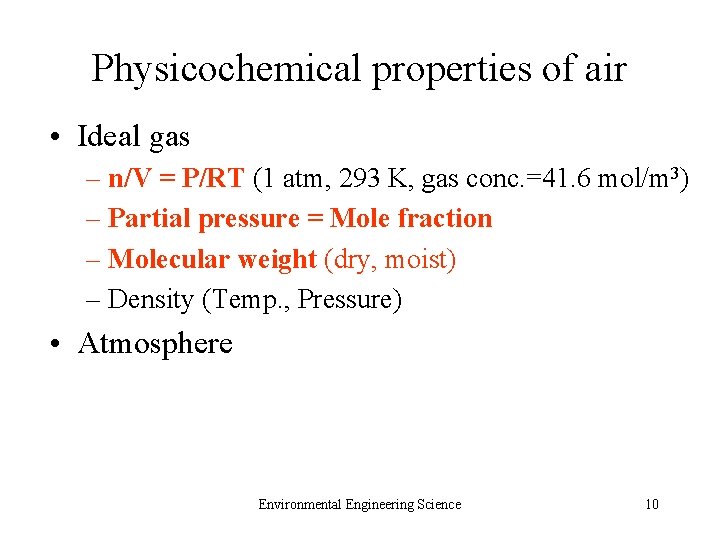
Physicochemical properties of air • Ideal gas – n/V = P/RT (1 atm, 293 K, gas conc. =41. 6 mol/m 3) – Partial pressure = Mole fraction – Molecular weight (dry, moist) – Density (Temp. , Pressure) • Atmosphere Environmental Engineering Science 10


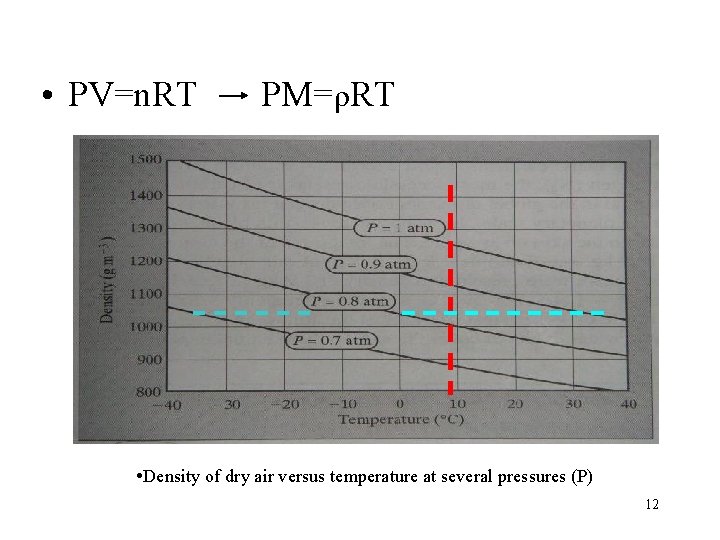
• PV=n. RT PM=ρRT • Density of dry air versus temperature at several pressures (P) 12

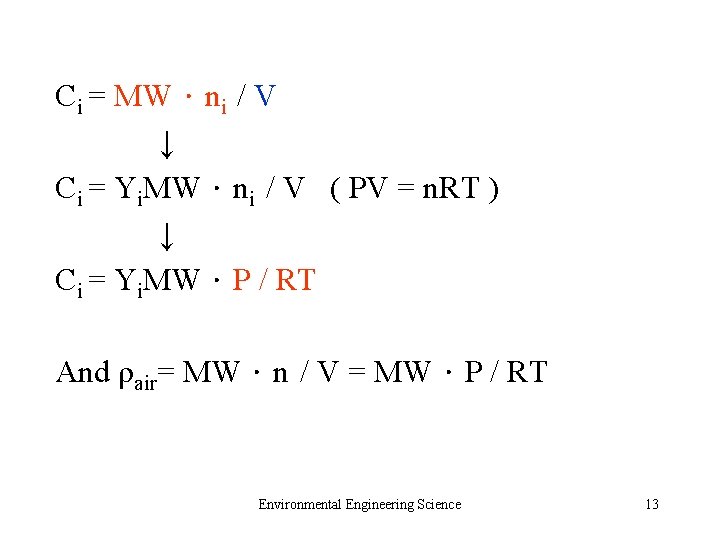
Ci = MW.ni / V ↓ Ci = Yi. MW.ni / V ( PV = n. RT ) ↓ Ci = Yi. MW.P / RT And ρair= MW.n / V = MW.P / RT Environmental Engineering Science 13

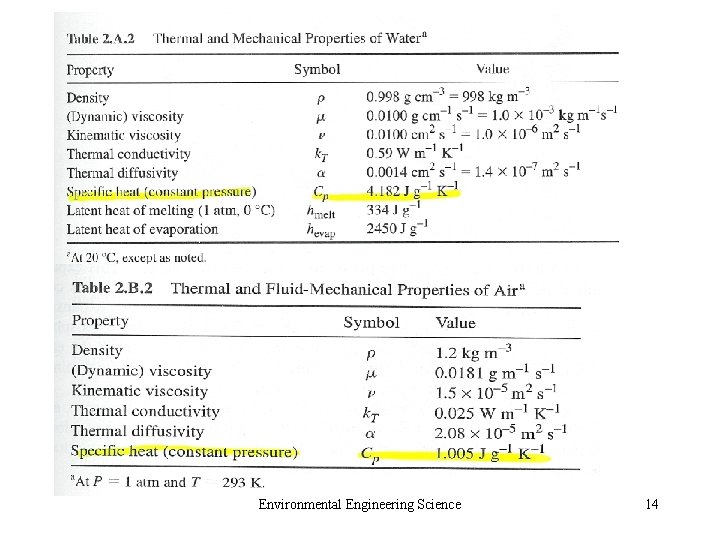
Environmental Engineering Science 14

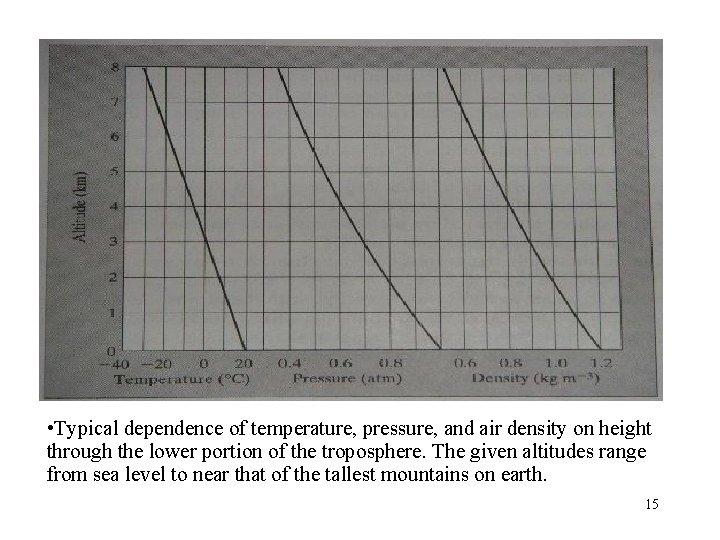
• Typical dependence of temperature, pressure, and air density on height through the lower portion of the troposphere. The given altitudes range from sea level to near that of the tallest mountains on earth. 15

2. C Impurities in Environmental Media – 2. C. 1 Gases Dissolved in water – 2. C. 2 Water in Air – 2. C. 3 Acids, Bases, and the Hydrogen Ion – 2. C. 4 Inorganic Impurities – 2. C. 5 Organic Impurities – 2. C. 6 Radio nuclides – 2. C. 7 Compounds Causing Odor, Taste, or Color – 2. C. 8 Particulate Matter – 2. C. 9 Microorganisms Environmental Engineering Science 16

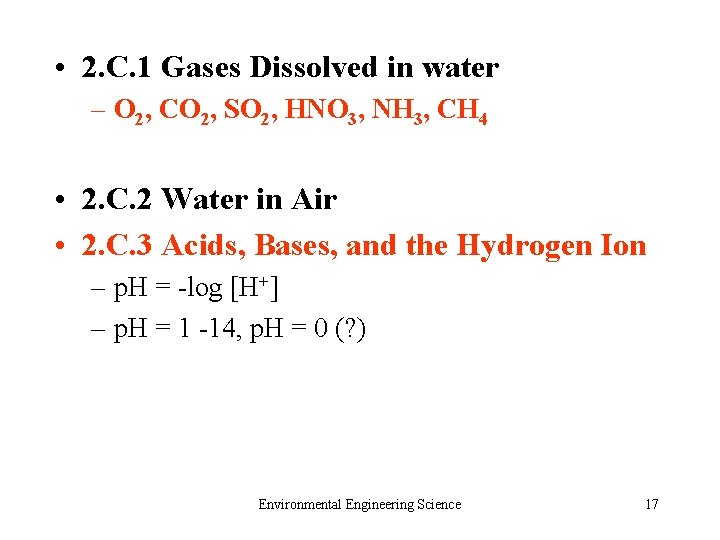
• 2. C. 1 Gases Dissolved in water – O 2, CO 2, SO 2, HNO 3, NH 3, CH 4 • 2. C. 2 Water in Air • 2. C. 3 Acids, Bases, and the Hydrogen Ion – p. H = -log [H+] – p. H = 1 -14, p. H = 0 (? ) Environmental Engineering Science 17

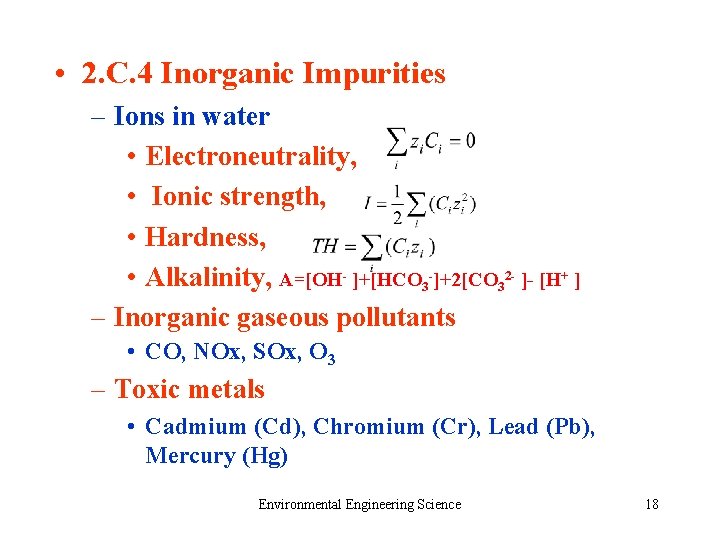
• 2. C. 4 Inorganic Impurities – Ions in water • Electroneutrality, • Ionic strength, • Hardness, • Alkalinity, A=[OH- ]+[HCO 3 -]+2[CO 32 - ]- [H+ ] – Inorganic gaseous pollutants • CO, NOx, SOx, O 3 – Toxic metals • Cadmium (Cd), Chromium (Cr), Lead (Pb), Mercury (Hg) Environmental Engineering Science 18

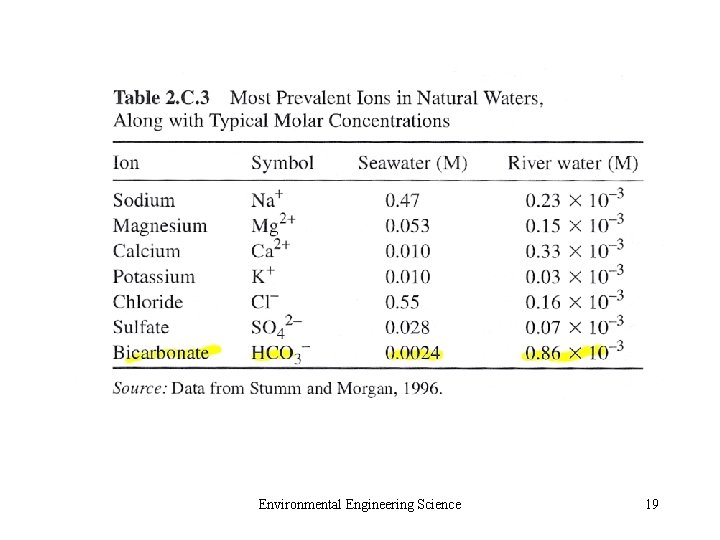
Environmental Engineering Science 19



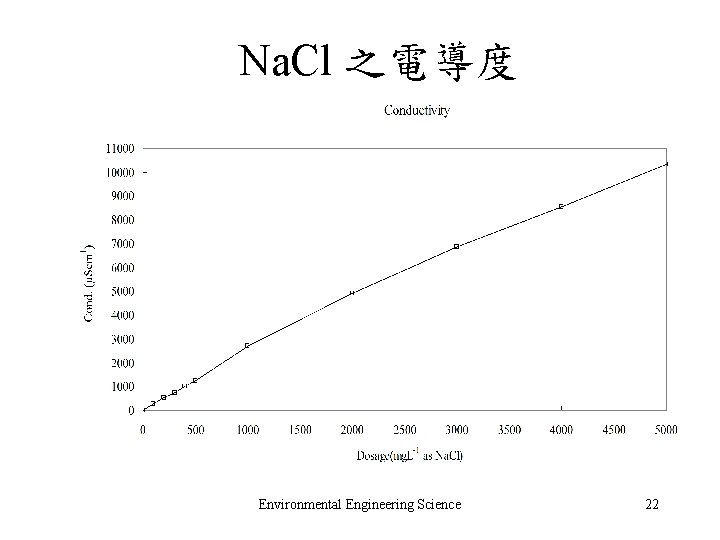
Na. Cl 之電導度 Environmental Engineering Science 22

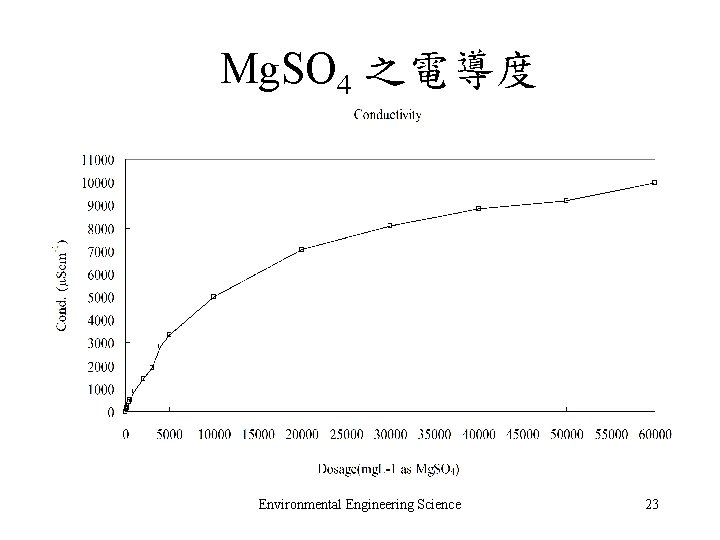
Mg. SO 4 之電導度 Environmental Engineering Science 23

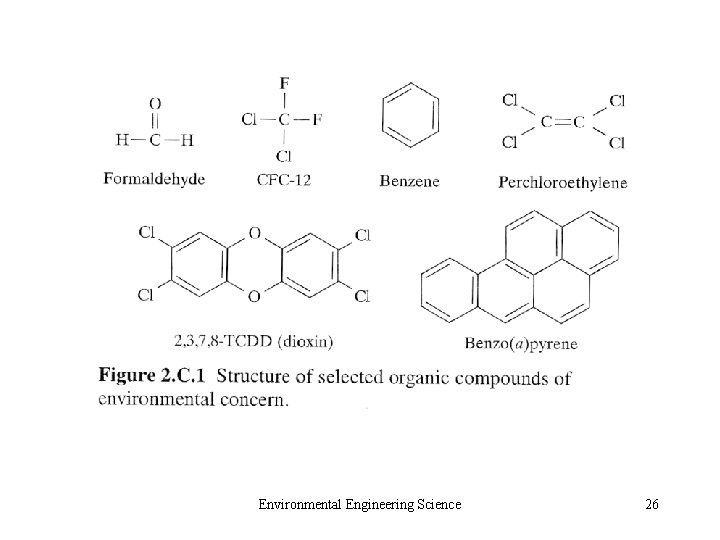
• 2. C. 5 Organic Impurities – Specific organic impurities • HCHO, CFCs, Benzene, PCE, Dioxin, PAHs – Aggregate measurement of organic compounds • OD, TOD (Th. OD), BOD, COD, TOC • NMHC Environmental Engineering Science 24


Environmental Engineering Science 26

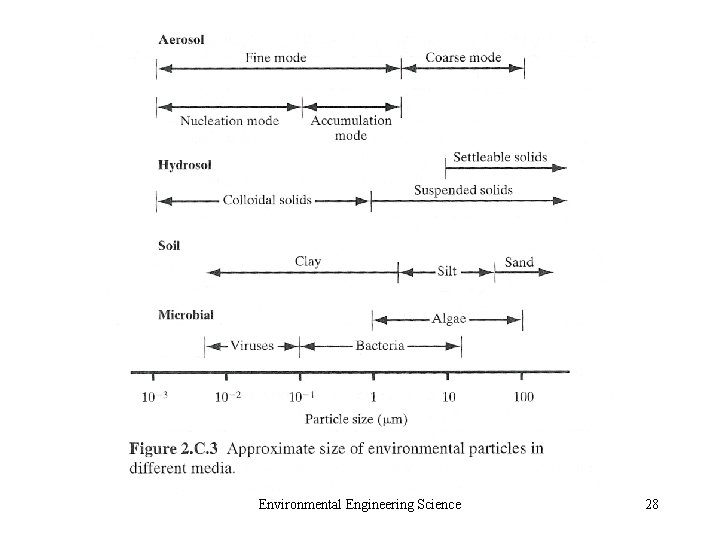
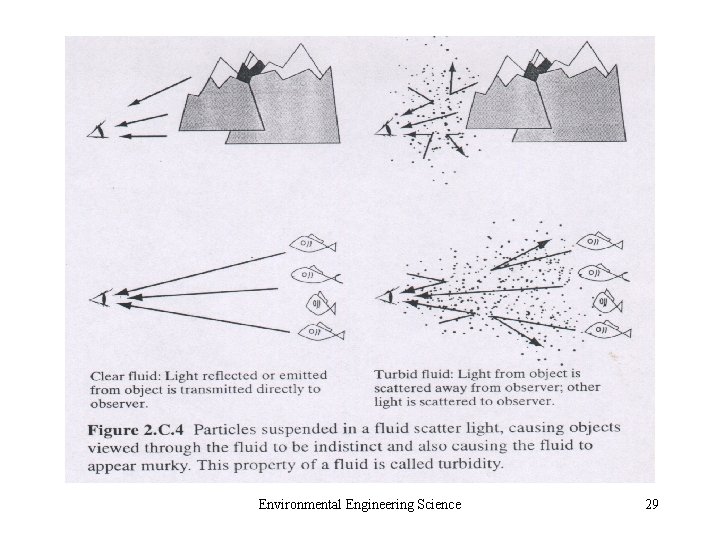
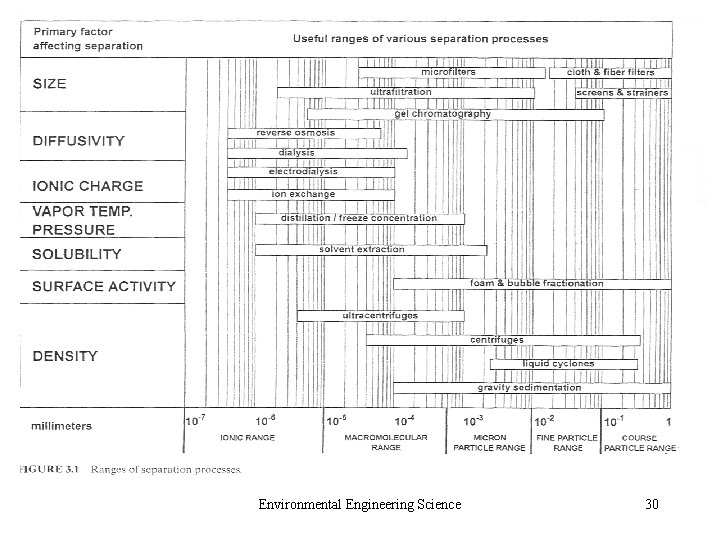
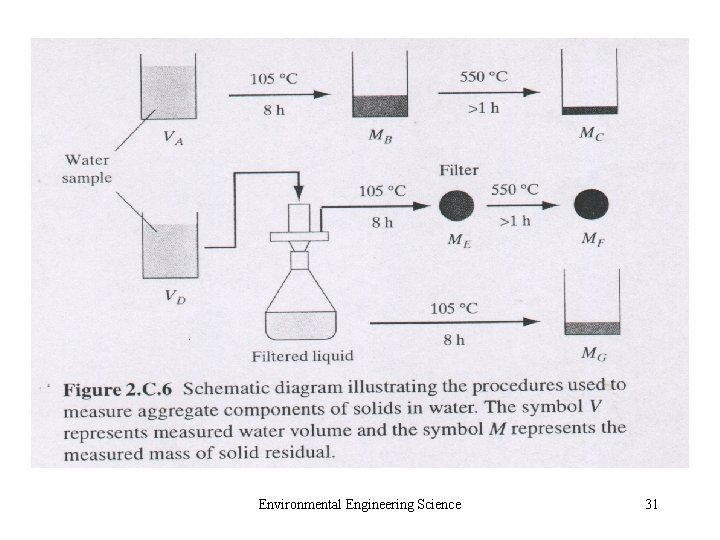
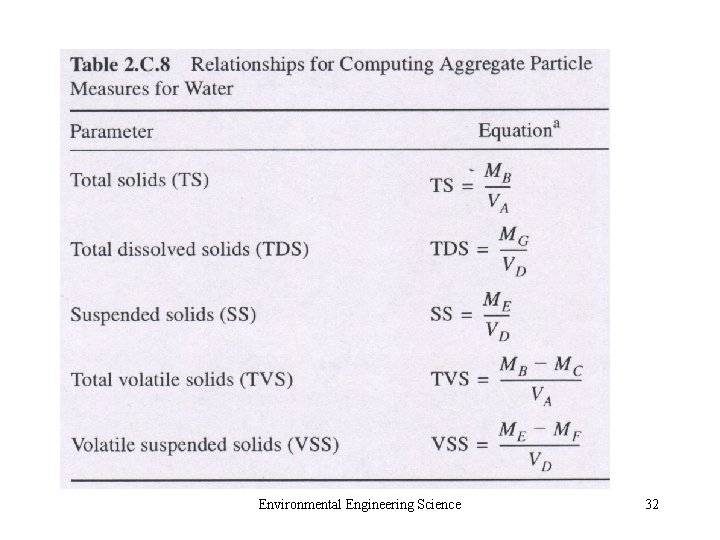
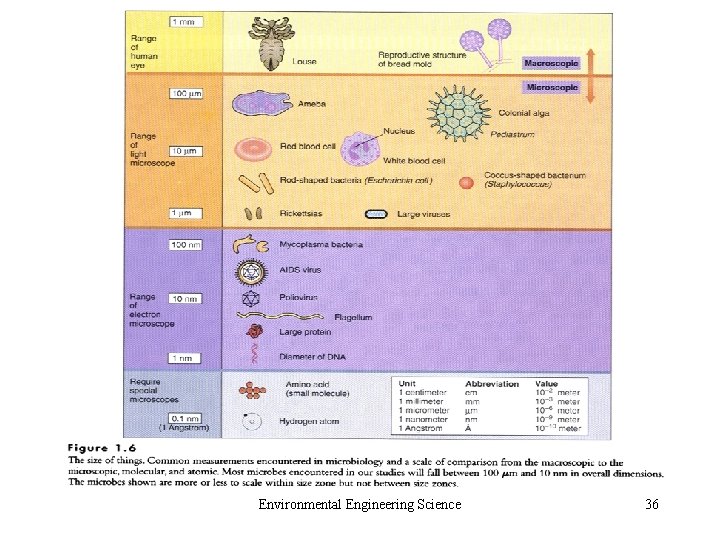
• 2. C. 6 Radio nuclides • 2. C. 7 Compounds Causing Odor, Taste, or Color • 2. C. 8 Particulate Matter – Aerosol and Hydrosol – Small Colloids (10 -3 μm) – Particles in Air: fine and coarse – Particles in Water: settleable, suspended and colloidal solid Environmental Engineering Science 27

Environmental Engineering Science 28

Environmental Engineering Science 29

Environmental Engineering Science 30

Environmental Engineering Science 31

Environmental Engineering Science 32

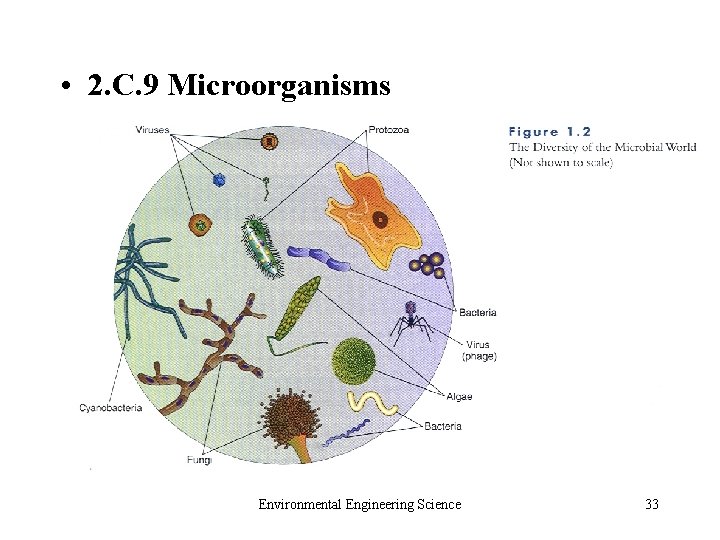
• 2. C. 9 Microorganisms Environmental Engineering Science 33


• Prokaryotic • Bacteria • Archaea • Viruses • Eukaryotic • Algae • Fungi • Protozoa

Environmental Engineering Science 36

Problem assignments #2 In textbook: • 2. 3 Volume occupied by air • 2. 14 Electroneutrality • 2. 15 River water quality • READING: Chapter 2: Section 2. C (pp. 41 -69) Chapter 3: Sections 3. A and 3. B (pp. 76 -106) Appendix C (pp. 613 -619 Environmental Engineering Science 37
 Water and water and water water
Water and water and water water Trivalent and pentavalent impurities
Trivalent and pentavalent impurities Air higroskopis adalah
Air higroskopis adalah Creole is a derivative sauce of
Creole is a derivative sauce of Structure and properties of ceramics
Structure and properties of ceramics Diffused impurities with five valence electrons are called
Diffused impurities with five valence electrons are called Example of donor impurities
Example of donor impurities Desvenlafaxine medscape
Desvenlafaxine medscape Vich gl 18
Vich gl 18 Characteristics of fronts
Characteristics of fronts Single acting cylinder example
Single acting cylinder example Is methane lighter than air
Is methane lighter than air A shuttle valve exhausts an air pilot of a dcv when
A shuttle valve exhausts an air pilot of a dcv when Pneumatic time delay valve symbol
Pneumatic time delay valve symbol Measures air temperature
Measures air temperature 2 causes of land pollution
2 causes of land pollution Soil pollution images diagram
Soil pollution images diagram Backrest comfort device
Backrest comfort device Air and water resources lesson 4
Air and water resources lesson 4 In fair verona where we lay our scene translation
In fair verona where we lay our scene translation Dock water allowance adalah
Dock water allowance adalah Air hujan sanggup menjadi air tanah lantaran proses
Air hujan sanggup menjadi air tanah lantaran proses Contoh pelarut protonik
Contoh pelarut protonik Chapter 12 section 1 what causes air pollution answers key
Chapter 12 section 1 what causes air pollution answers key Can you feel it in the air in the air
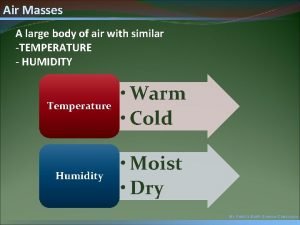
Can you feel it in the air in the air A large body of air
A large body of air Two cold air masses converge on a warm air mass
Two cold air masses converge on a warm air mass Right hand in the air
Right hand in the air All kinds of shapes
All kinds of shapes Right hand in the air left hand in the air
Right hand in the air left hand in the air Air payau adalah campuran dari
Air payau adalah campuran dari Shell and tube heat exchanger in food industry
Shell and tube heat exchanger in food industry Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Actual air refrigeration system
Actual air refrigeration system Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Pekerjaan sanitasi
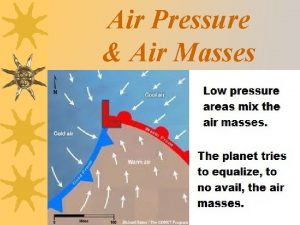
Pekerjaan sanitasi A swirling center of low air pressure is called
A swirling center of low air pressure is called