WATER FOR LIFE SOURCES OF WATER IMPURITIES WATER


























































































- Slides: 109

WATER FOR LIFE SOURCES OF WATER IMPURITIES WATER OF WATER POLLUTION

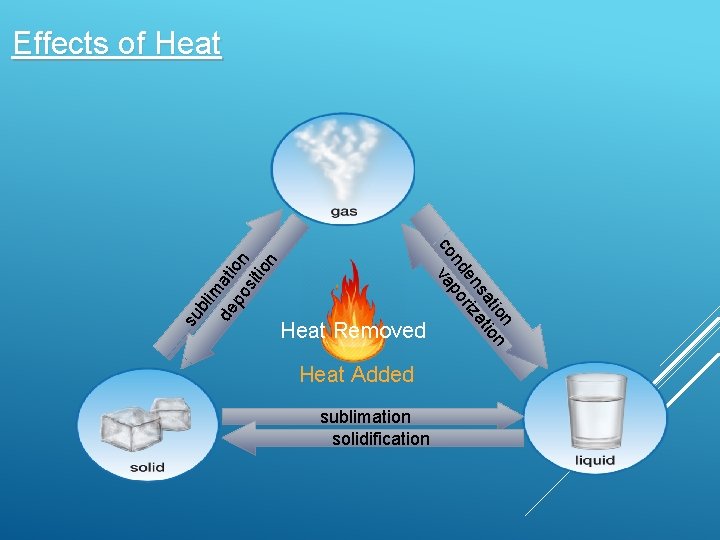
Changes of State ü We will be looking at changes between the 3 common states of matter. ü There actually six changes of state that can happens as a substance switches from one physical form to another su bli m at ion de po sit ion at n io at riz po va ns de n co sublimation solidification

Heat Removed Heat Added sublimation solidification n tio on sa ati en riz nd po co va su bli m de ati po on sit ion Effects of Heat

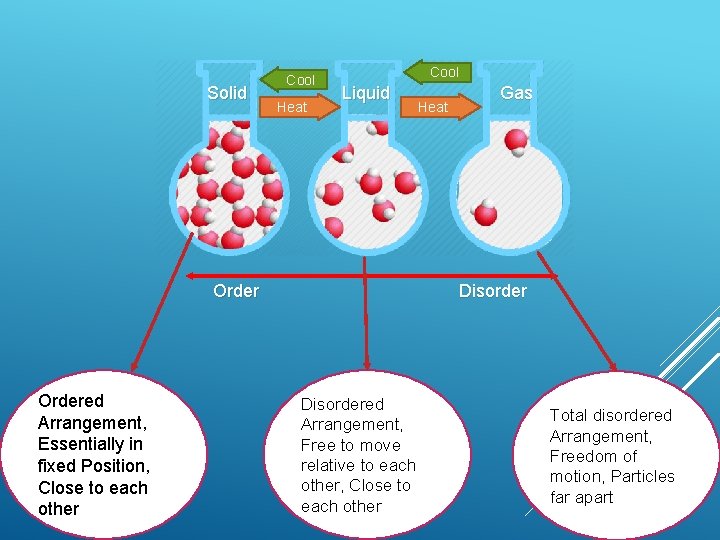
Solid Cool Heat Cool Liquid Ordered Arrangement, Essentially in fixed Position, Close to each other Heat Gas Disordered Arrangement, Free to move relative to each other, Close to each other Total disordered Arrangement, Freedom of motion, Particles far apart

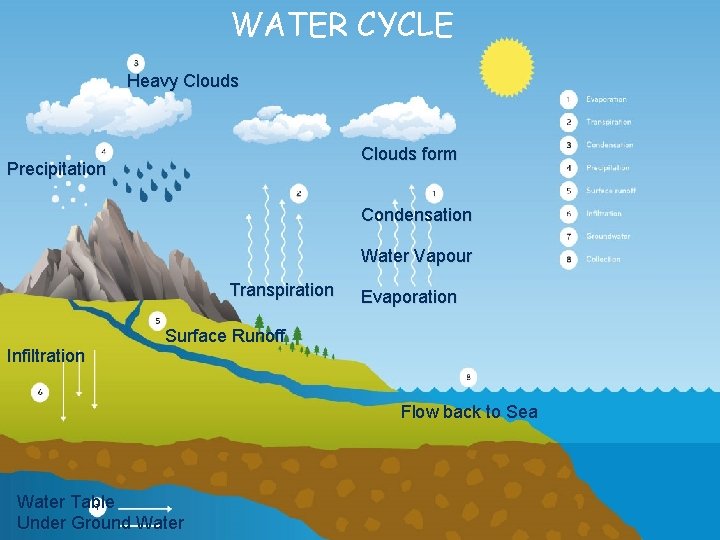
WATER CYCLE Heavy Clouds form Precipitation Condensation Water Vapour Transpiration Infiltration Evaporation Surface Runoff Flow back to Sea Water Table Under Ground Water

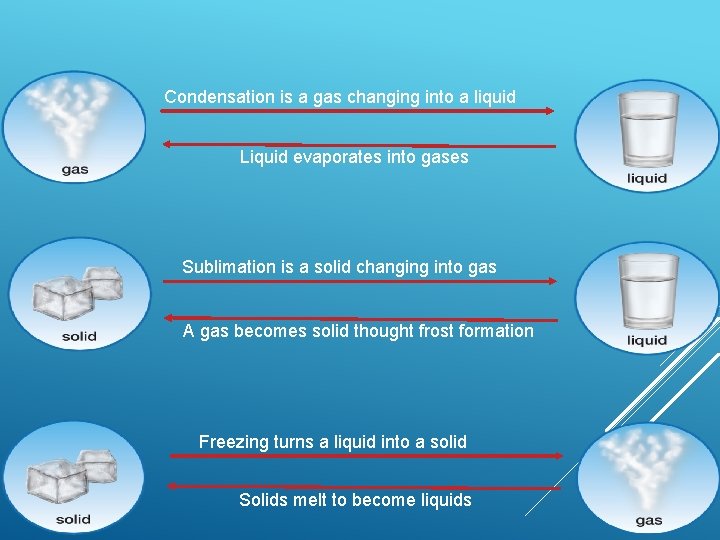
Condensation is a gas changing into a liquid Liquid evaporates into gases Sublimation is a solid changing into gas A gas becomes solid thought frost formation Freezing turns a liquid into a solid Solids melt to become liquids

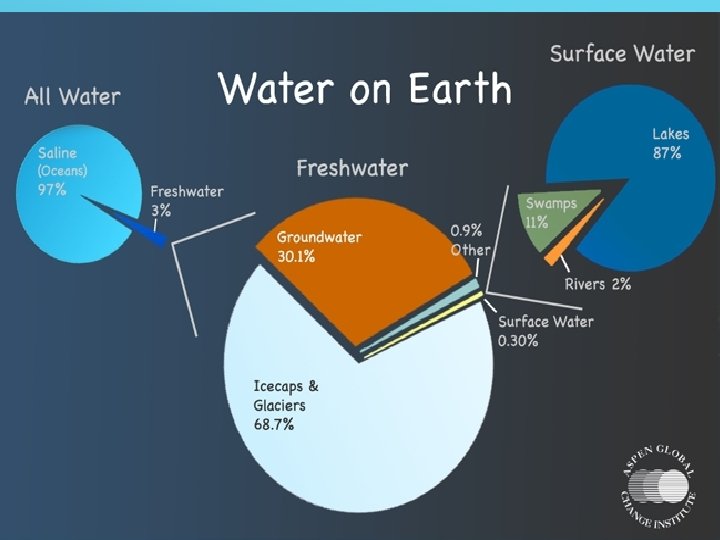
OCCURRENCE OF WATER

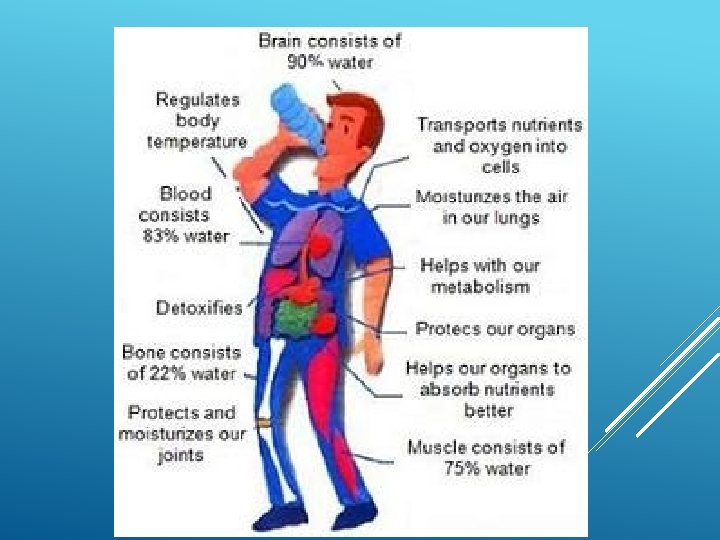
IMPORTANCE OF WATER


SOURCES OF WATER




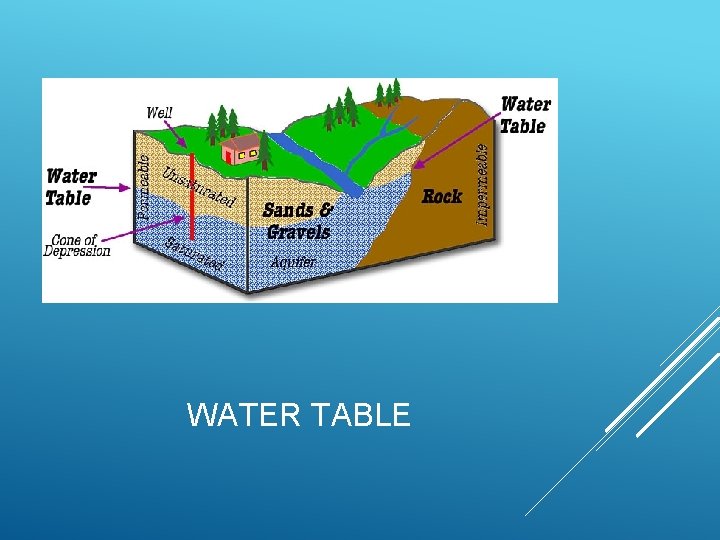
Aquifer is from the Latin aqua (meaning "water") and ferre (meaning "to bear") –– an aquifer literally bears water. An aquifer is an underground layer of waterbearing rock. Water-bearing rocks are permeable, meaning that they have openings that liquids and gases can pass through. Sedimentary rock as well as sand gravel, are examples of water-bearing rock. The top of the water level in an aquifer is called the water AQUIFER table.

PURPOSE: Aquifers act as reservoirs for groundwater. Water from aquifers sometimes flows out in springs. Wells drilled into aquifers provide water for drinking, agriculture, and industrial uses.

Aquifers can dry up when people drain them faster than nature can refill them. Because aquifers fill with water that drains from the surface of the Earth, they can be contaminated by any chemical or toxic substance found on the surface.

DEFINITIONS OF AQUIFER 1. a layer of rock or sand that can absorb and hold water 2. An underground layer of permeable rock, sediment, or soil that yields water. Aquifers can range from a few square kilometers to thousands of square kilometers in size.


GLACIERS Definition: ‘A large mass of ice moving very slowly through a valley or spreading outward from a center. ’ Formation: Glaciers form over many years from packed snow in areas where snow accumulates faster than it melts. A glacier is always moving, but when its forward edge melts faster than the ice behind it advances, the glacier as a whole shrinks backward.

GLACIERS


ICE SHEETS

‘An ice sheet is a mass of glacial land ice extending more than 50, 000 square kilometers (20, 000 square miles). ’ DEFINITION OF ICE SHEET

ICE CAPS

DEFINITION OF ICE CAPS ‘Ice caps are miniature ice sheets, covering less than 50, 000 square kilometers (19, 305 square miles). ’

WETLANDS


Definition: ‘A wetland is a land area that is saturated with water, either permanently or seasonally, such that it takes on the characteristics of a distinct ecosystem. ’ Primarily, the factor that distinguishes wetlands from other land forms or water bodies is the characteristic vegetation of aquatic plants, adapted to its unique hydric soil.

GROUND WATER Definition: ‘Water that flows or collects beneath the Earth's surface. ’ Origin: Groundwater originates from rain and from melting snow and ice. It sinks into the ground, filling the small empty spaces in soil, sediment, and porous rocks. Aquifers, springs, and wells are supplied by the flow of groundwater.

WATER TABLE

Definition: ‘Water pollution is the contamination of water bodies (e. g. lakes, rivers, oceans, aquifers and groundwater). ’ This form of environmental degradation occurs when pollutants are directly or indirectly discharged into water bodies WATER POLLUTION without adequate treatment to remove harmful compounds.



‘The addition of harmful substances into the water is called water pollution. ’ ‘Harmful and unwanted substances in water are called pollutants. ’

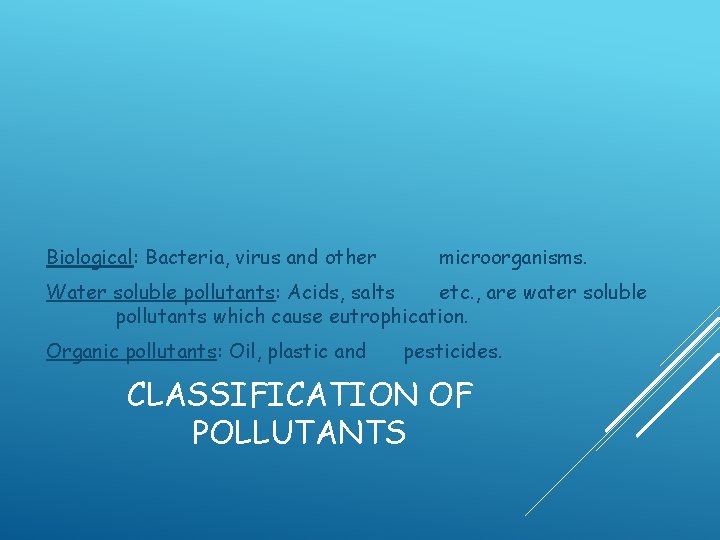
Biological: Bacteria, virus and other microorganisms. Water soluble pollutants: Acids, salts etc. , are water soluble pollutants which cause eutrophication. Organic pollutants: Oil, plastic and pesticides. CLASSIFICATION OF POLLUTANTS

Cause: Water pollution occurs when harmful substances are released into the water in large quantities which cause damage to people, wildlife, or habitat or indirectly into water bodies without proper treatment to remove harmful compounds. Effects: Water pollution affects plants and organisms living in the bodies of water; and, in almost all cases the effect is damaging either to individual species and also to the biological communities.


EFFECTS OF WATER POLLUTION IN DETAIL

GROUNDbetween WATER POLLUTION Interactions groundwater and surface water are complex. However some of the polluting agents are as follows: Infectious Agents: bacteria and viruses often from animal wastes Oxygen Demanding Wastes: organic waste that needs oxygen often from animal waste, paper mills and food processing. Inorganic Chemicals: Acids and toxic chemicals often from runoff, industries and household leaners

HOW DO WEis. MEASURE Water quality determined WATER by QUALITY? measuring its BOD: Biological Oxygen Demand… the amount of oxygen consumed by aquatic decomposers.

WATER POLLUTION

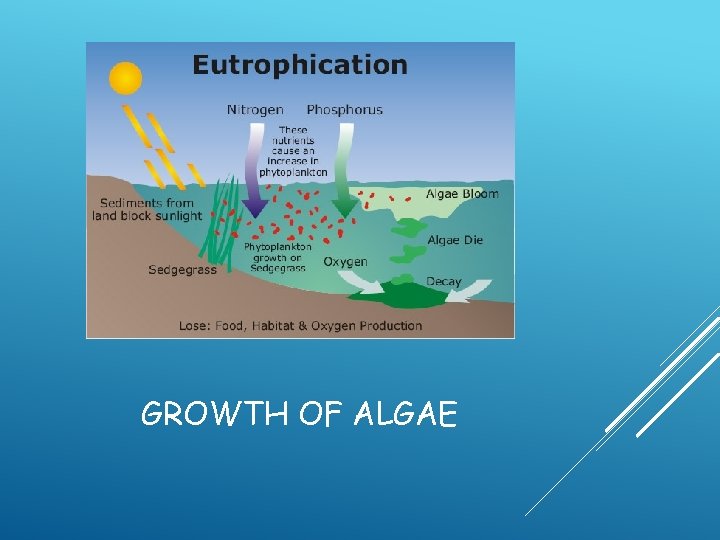
GROWTH OF ALGAE

Definition: The process by which a body of water becomes enriched in dissolved nutrients (as phosphates) that stimulate the growth of aquatic plant life usually resulting in the depletion of dissolved oxygen. EUTROPHICATION

EUTROPHICATION OF LAKES Eutrophication: nutrient enrichment of lakes mostly from runoff of plant nutrients (nitrates and phosphates). During hot dry weather can lead to algae blooms Decrease of photosynthesis Dying algae then drops DO levels Fish kills, bad odor


1. Human Wastes WHAT IS DOMESTIC SEWAGE? Sewage originating primarily from kitchen, bathroom, and laundry sources. Waste from food preparation, dishwashing, garbagegrinding, toilets, baths, showers, and sinks. SOURCES OF WATER POLLUTION

WHY IS DOMESTIC SEWAGE A PROBLEM? Domestic sewage contains a wide variety of dissolved and suspended impurities. It amounts to a very small fraction of the sewage by weight, but it is large by volume and contains impurities such as organic materials and plant nutrients that tend to rot. The main organic materials are food and vegetable wastes. Plant nutrients come from chemical soaps, washing powders, etc.

WHEN SEWAGE ENTERS A LAKE OR STREAM, MICROORGANISMS BEGIN TO DECOMPOSE THE ORGANIC MATERIALS. OXYGEN IS CONSUMED AS MICRO-ORGANISMS USE IT IN THEIR METABOLISM

DAMAGE Sewage-contaminated water causes eutrophication, which is the increase in concentration of chemical elements required for life. The nitrates, phosphates, and organic matter found in human waste serves as a food for algae and bacteria. This causes these organisms to overpopulate to the point where they use up most of the dissolved oxygen that is naturally found in water, making it difficult for other organisms in this aquatic environment to live.

Health Risks Bathers are at increased risk of contracting illness due to bacteria and viruses present in sewage effluent. Gastrointestinal disorders have been linked to sewage pollution, with viruses implicated as the cause.

Certain fish in contaminated waters can accumulate high levels of toxic (poisonous) substances. When these foods are consumed frequently over a lifetime, they may increase the consumers’ risk of adverse health effects. Detergents can cause liver and kidney damage, while sewage water carries diseases such as dysentery and Cholera.

How do we get infected by contaminated water? oral - drinking contaminated water or eating contaminated seafood dermal - getting contaminated water on your skin and in open cuts or rashes aerosol - inhaling water droplets such as those from breaking waves.

Industry is a huge source of water pollution, it produces pollutants that are extremely harmful to people and the environment. INDUSTRIAL WASTE





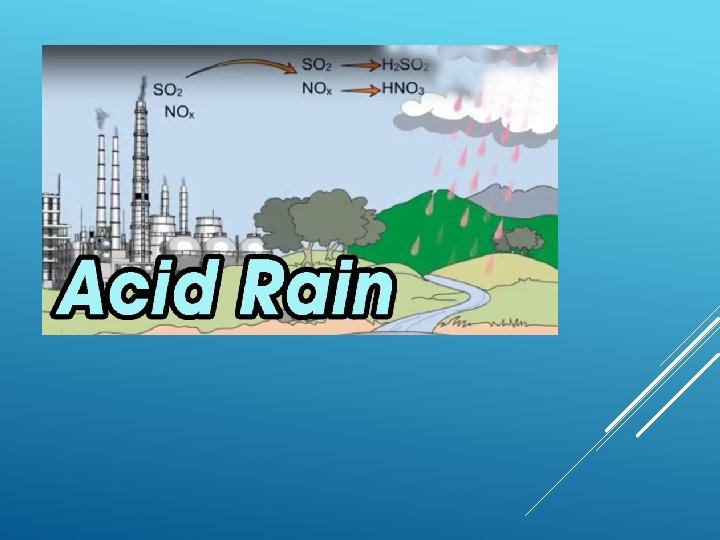
ACID RAIN


Precipitation, as rain, snow, containing relatively high concentrations of acid-forming chemicals, as the pollutants from coal smoke and chemical industries that have been released into the atmosphere and combined with water vapour harmful to the environment. ACID RAIN


CAUSES OF ACID RAIN Burning coal, Oil and natural gas in power stations makes electricity, giving off sulphur dioxide gas. Burning petrol and oil in vehicle engines gives off nitrogen oxides as gases. These gases mix with water vapour and rainwater in the atmosphere producing weak solutions of sulphuric and nitric acids – which fall as acid rain.

Acid rain can travel long distances. Often it doesn’t fall where the gas is produced. High chimneys disperse (spread) the gases and winds blow them great distances before they dissolve and fall to Earth as rain, e. g. gases produced in England Western Europe can result in acid rain in Scotland Scandinavia.

HOW ACID RAIN AFFECTS THE ENVIRONMENT Acid rain is an extremely destructive form of pollution, and the environment suffers from its effects. Forests, trees, lakes, animals, and plants suffer from acid rain. Trees The needles and leaves of the trees turn brown and fall off. Trees can also suffer from stunted growth; and have damaged bark and leaves, which makes them vulnerable to weather, disease, and insects.


All of this happens partly because of direct contact between trees and acid rain, but it also happens when trees absorb soil that has come into contact with acid rain. The soil poisons the tree with toxic substances that the rain has deposited into it.

LAKES are also damaged by acid rain. Fish die off, and that removes the main source of food for birds. Fish usually die only when the acid level of a lake is high; when the acid level is lower, they can become sick, suffer stunted growth, or lose their ability to reproduce. Also, birds can die from eating "toxic" fish and insects.


Acid rain dissolves the stonework and mortar of buildings (especially those made out of sandstone or limestone). It reacts with the minerals in the stone to form a powdery substance that can be washed away by rain. BUILDINGS



WHAT ARE THE SOLUTIONS TO 1. Sulphur dioxide can be removed from power ACID RAIN? stations chimneys but this process is expensive. 2. Use renewable energy like wind power, solar panels. 3. Fit catalytic converters to vehicle exhausts which remove the nitrogen oxides. 4. Limit the number of vehicles on the roads and increase public transport.


SOFT AND HARD WATER

SOFT WATER The water that lathers with soap easily is called soft water. It describes type of water that contain few or no minerals like calcium (Ca) or magnesium (Mg) ions.

Water that contains mineral salts (as calcium and magnesium ions) that limit the formation of lather with soap. HARD WATER

Water where you live might be hard or soft – this depends on the rocks the water meets on its way to you… DIFFERENCE

Hard water causes scum rather than a nice lather with soap – this is due to dissolved calcium and magnesium ions in the water which react with the soap making it insoluble When heated hard water forms scales in most parts which is calcium carbonate – this affects the efficiency of kettles and boilers (as scale is a thermal insulator)

Most hard water contains lots of calcium ions and magnesium ions from the rocks it has passed through (such as limestone and chalk)


Purification of water involves physical, biological and chemical processes such as: 1. Filtration 2. Chlorination 3. Boiling 4. Use of potash Alum 5. Distillation PURIFICATION OF WATER

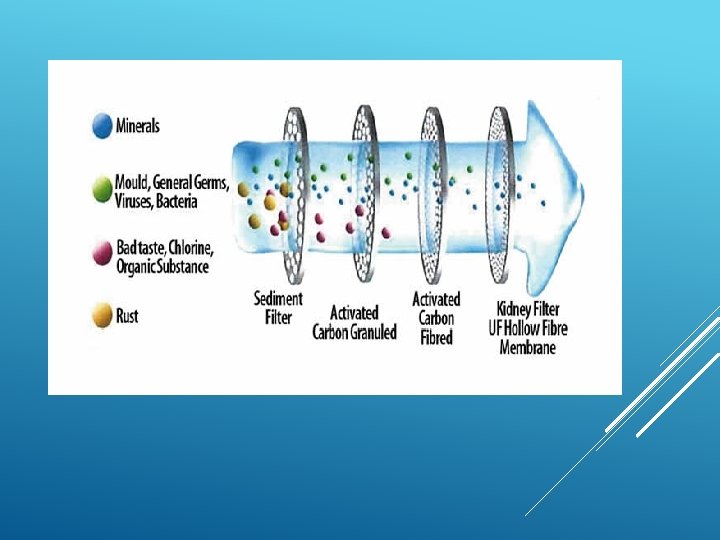
WATER FILTRATION BACKGROUND: Water in lakes, rivers, and swamps often contains impurities that make it look and smell bad. The water may also contain bacteria and other microbiological organisms that can cause disease. Consequently, water from most surface sources must be “cleaned” before it can be consumed by people. PHYSICAL PROCESSES

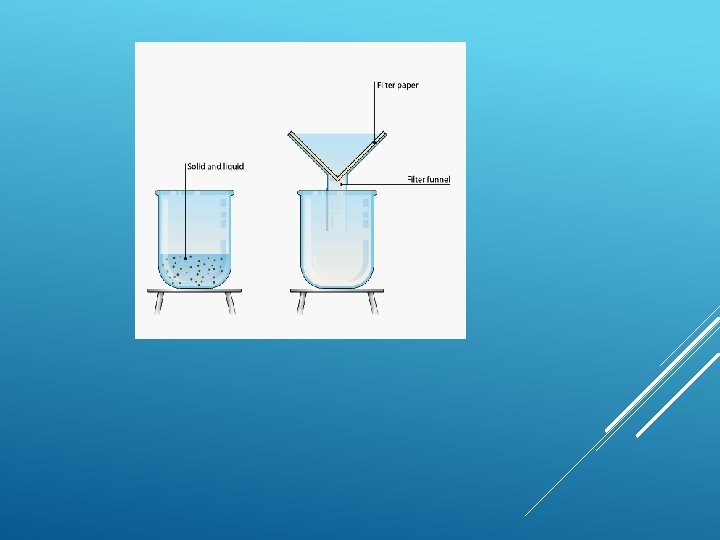
FILTRATION Filtration is good for separating an insoluble solid from a liquid. (An insoluble substance is one that does not dissolve). Sand, for example, can be separated from a mixture of sand water using filtration. That's because sand does not dissolve in water.




To remove dissolved substances from water, special membranes are used which have microscopic pores to separate dissolved substances from water.



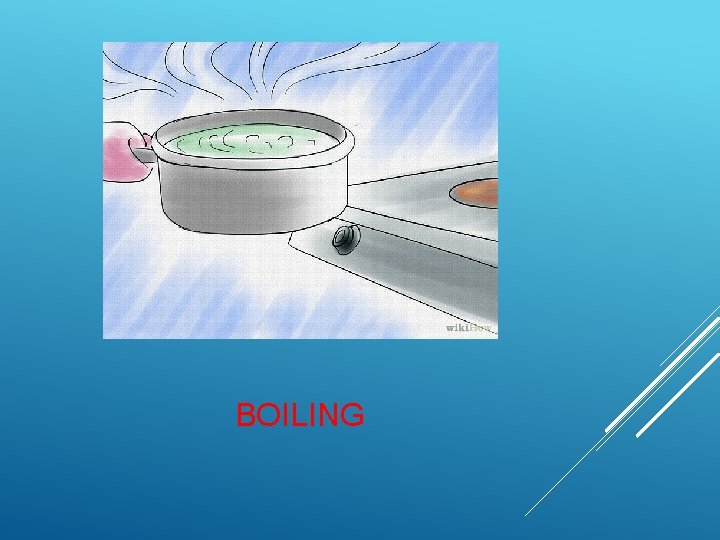
BOILING

Place the water you wish to purify into a pot. Place the pot on the stove and turn the stove on to high. When water boils, any bacteria that may have been living in it will be killed, thus reducing your chance of getting sick when you drink the water.

Boiling water for 15 to 30 minutes will purify it sufficiently. It will kill off the vast majority of organisms living in the water. It also removes some chemicals by vaporizing them. However, be aware that boiling the water will not remove solids, metals, or minerals.

Disinfection of water by the addition of small amounts of chlorine or a chlorine compound. Disinfection ‘Treatment to destroy harmful microorganisms. ’ CHLORINATION



USE OF POTASH ALUM

"By the addition of a small amount of alum to water, it can be filtered through ordinary paper without difficulty, and yields a brilliantly clear water, in which there is no trace of suspended matter. In experiment, a solution of alum is added to the water, the whole well mixed by stirring or shaking, and then filtered after standing from one to fifteen minutes.

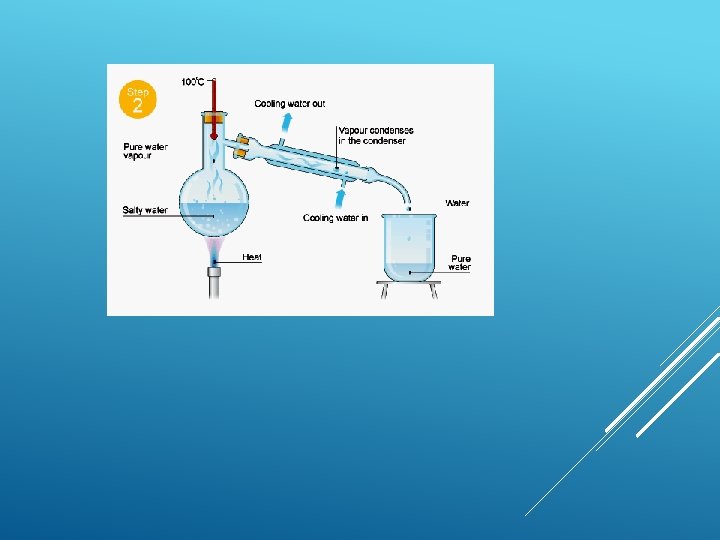
Distillation is defined as ‘a refining process that collects the pure liquid or vapors given off by heating mixture and then cooling vapors’. DISTILLATION

Simple distillation This is good for separating a liquid from a solution. For example, water can be separated from salty water by simple distillation. This method works because the water evaporates from the solution, but is then cooled and condensed into a separate container. The salt does not evaporate and so it stays behind.




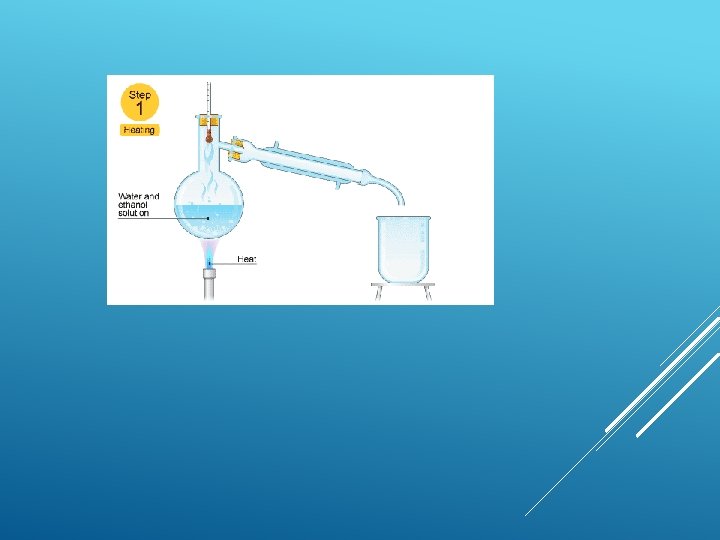
Fractional distillation This is good for separating two or more liquids from each other. For example, ethanol (alcohol) can be separated from a mixture of ethanol and water by fractional distillation. This method works because the two liquids have different boiling points.





Please save water. If you see anyone playing or polluting water stop them and make the world a better place to live in.

 Explain important of watershed management
Explain important of watershed management Print sources of information
Print sources of information The process of eliminating bones or impurities
The process of eliminating bones or impurities Structures and properties of ceramics
Structures and properties of ceramics The diffused impurities with
The diffused impurities with Example of donor impurities
Example of donor impurities Pristiq rxlist
Pristiq rxlist Trivalent and pentavalent impurities
Trivalent and pentavalent impurities Common solvent impurities
Common solvent impurities Water and water and water water
Water and water and water water Fspos
Fspos Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Tidböcker
Tidböcker Anatomi organ reproduksi
Anatomi organ reproduksi Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Debattartikel struktur
Debattartikel struktur För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Vätsketryck formel
Vätsketryck formel Offentlig förvaltning
Offentlig förvaltning Jag har gått inunder stjärnor text
Jag har gått inunder stjärnor text Presentera för publik crossboss
Presentera för publik crossboss Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Bat mitza
Bat mitza Treserva lathund
Treserva lathund Epiteltyper
Epiteltyper Bästa kameran för astrofoto
Bästa kameran för astrofoto Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Lågenergihus nyproduktion
Lågenergihus nyproduktion Bra mat för unga idrottare
Bra mat för unga idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referat mall
Referat mall Redogör för vad psykologi är
Redogör för vad psykologi är Stål för stötfångarsystem
Stål för stötfångarsystem Atmosfr
Atmosfr Borra hål för knoppar
Borra hål för knoppar Orubbliga rättigheter
Orubbliga rättigheter Standardavvikelse formel
Standardavvikelse formel Tack för att ni har lyssnat
Tack för att ni har lyssnat Rita perspektiv
Rita perspektiv Verksamhetsanalys exempel
Verksamhetsanalys exempel Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Blomman för dagen drog
Blomman för dagen drog Modell för handledningsprocess
Modell för handledningsprocess Egg för emanuel
Egg för emanuel Elektronik för barn
Elektronik för barn Antika plagg
Antika plagg Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Var 1721 för stormaktssverige
Var 1721 för stormaktssverige Humanitr
Humanitr Ro i rom pax
Ro i rom pax Tack för att ni lyssnade
Tack för att ni lyssnade Vilket tal pekar pilen på
Vilket tal pekar pilen på Bunden dikt
Bunden dikt Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Rådet för byggkompetens
Rådet för byggkompetens Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Kolposkopi, px
Kolposkopi, px Myndigheten för delaktighet
Myndigheten för delaktighet Frgar
Frgar Sju principer för tillitsbaserad styrning
Sju principer för tillitsbaserad styrning Läkarutlåtande för livränta
Läkarutlåtande för livränta Karttecken stig
Karttecken stig Skapa med geometriska former
Skapa med geometriska former Shivaismen
Shivaismen Var finns arvsanlagen
Var finns arvsanlagen Bris för vuxna
Bris för vuxna Jätte råtta
Jätte råtta Sanitary well parapet wall
Sanitary well parapet wall Non-point source pollution
Non-point source pollution 10 sources of water
10 sources of water Sources of water
Sources of water Predict the sources of water
Predict the sources of water City life and country life paragraph
City life and country life paragraph Farm life vs city life
Farm life vs city life Polynomial equation real life examples
Polynomial equation real life examples Single life is better
Single life is better Life orientation skills
Life orientation skills Country life vs city life compare /contrast
Country life vs city life compare /contrast City life vs country life
City life vs country life Moral lesson of life of pi
Moral lesson of life of pi Boundaries meme
Boundaries meme Life that is truly life
Life that is truly life John needham experiment main idea
John needham experiment main idea Unit 8 country life and city life
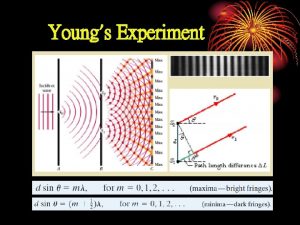
Unit 8 country life and city life First minima in diffraction
First minima in diffraction Sources and uses of funds
Sources and uses of funds How to cite many authors
How to cite many authors Esci web of science
Esci web of science Sources of groundwater pollution
Sources of groundwater pollution What are wastes
What are wastes Urea amino acids
Urea amino acids Steps in hypothesis testing ppt
Steps in hypothesis testing ppt Sources of personality
Sources of personality Lindisfarne raid primary sources
Lindisfarne raid primary sources Natural sound source theory of the origin of language
Natural sound source theory of the origin of language Real macbeth
Real macbeth Kesalahan dalam analisis data
Kesalahan dalam analisis data