Water Impurities and Purification 1 Continuing with Dissolved












![TSP Increases p. H �Na 3 PO 4 (TSP) ◦ Increases[PO 43−] needed to TSP Increases p. H �Na 3 PO 4 (TSP) ◦ Increases[PO 43−] needed to](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-22.jpg)
![DSP Maintains p. H �Na 2 HPO 4 (DSP) ◦ Increases [PO 43−] needed DSP Maintains p. H �Na 2 HPO 4 (DSP) ◦ Increases [PO 43−] needed](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-23.jpg)
![MSP Decreases p. H �Na. H 2 PO 4 (MSP) ◦ Increases [PO 43−] MSP Decreases p. H �Na. H 2 PO 4 (MSP) ◦ Increases [PO 43−]](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-24.jpg)
- Slides: 26

Water Impurities and Purification 1

Continuing with Dissolved Solids �Last week we looked at systems for treating external water for water hardness: ◦ Lime – soda softening ◦ Sodium zeolite softening ◦ Demineralizer �We will look at reverse osmosis as another method for removing dissolved solids from external water ◦ First we will look at osmosis 2

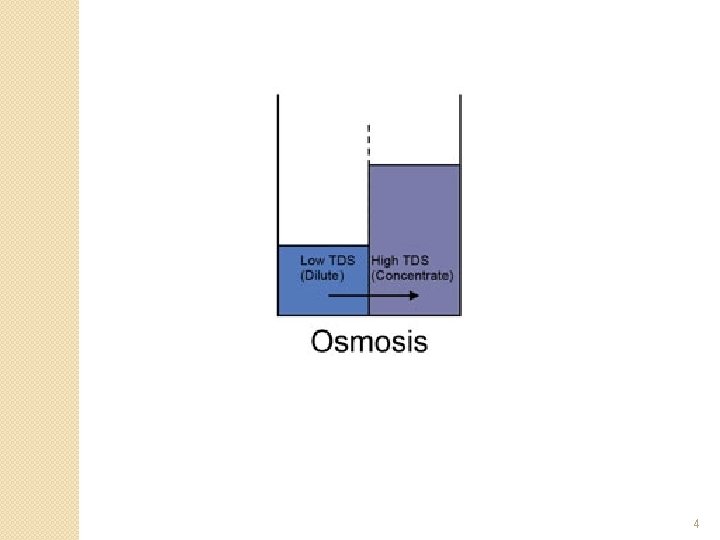
What is Osmosis �When a semi-permeable membrane separates two solutions, with different concentrations of dissolved solids, the solvent (water) from the dilute solution will diffuse through the membrane into the more concentrated solution. ◦ This is to equalize the concentration on both sides of the membrane 3

4

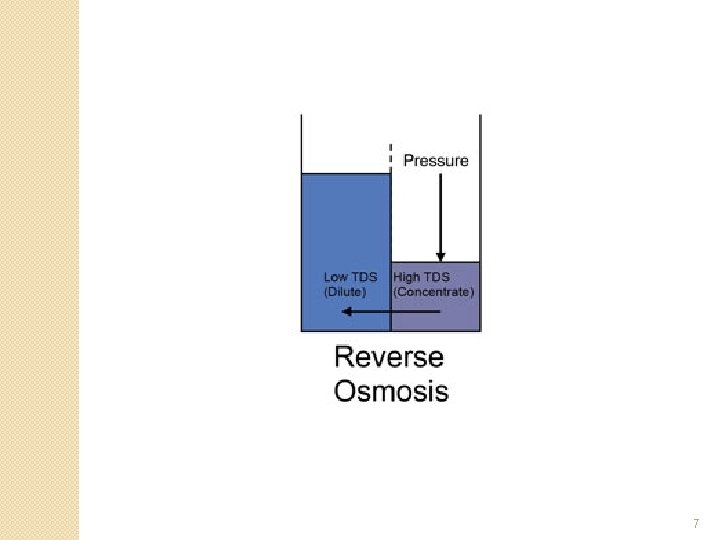
Reverse Osmosis �Osmosis is reversible with the application of high pressure on the concentrated solution and is known as reverse osmosis. �Water, with a high concentration of suspended solids, is pressurized and forced through a number of membrane modules connected in parallel. The impurities are left behind and the water on the low pressure side will have a high purity. 5

Reverse Osmosis �The raw water may be pretreated first ◦ filtration or settling �The water is then pumped through the membrane modules or permeators, at high pressure. ◦ Pressures greater than 1000 k. Pa �to over come the high osmotic pressures �dependant on the type of the membrane used. �Reverse osmosis can achieve up to 99% removal of dissolved salts from water. 6

7

Dissolved Gases �Dissolved gases refers to O 2, N 2 and CO 2 gases that are dissolved in the boiler water or steam ◦ non-condensable ◦ increase pressure in condensers decreasing operating efficiency and CO 2 also cause corrosion of boiler tube metals �O 2 8

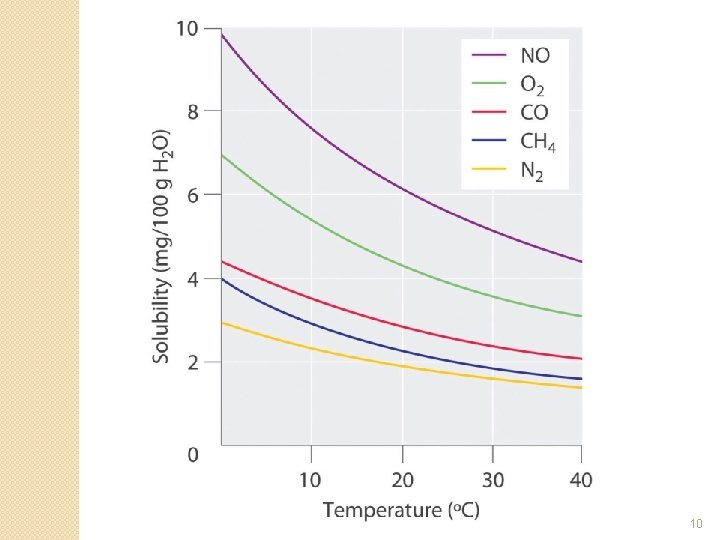
Removing Dissolved Gases – Mechanical Deaeration �Mechanical deaeration is used to removed dissolved gases in the internal feedwater ◦ The water is heated to near boiling point �Gas solubility decreases with temperature (see next slide) �due to the increased kinetic energy of the gas molecules �the molecules overcome intermolecular bonds and escape the solution ◦ The water is scrubbed with steam ◦ The gases are vented out along with the steam 9

10

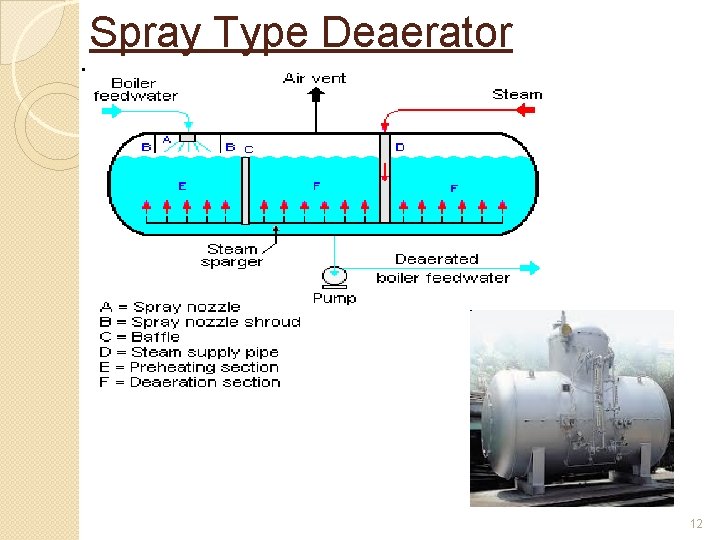
Spray Type Deaerator �The water passes through spray nozzles ◦ changes water into a fine mist. �In the preheating section ◦ the water is heated by contact with steam. �The water then passes through a baffle into the deaeration section ◦ scrubbed by steam ◦ released gases are removed by venting. �Deaerated water is pumped from the bottom of the deaerator into the boiler. �See next slide 11

Spray Type Deaerator 12

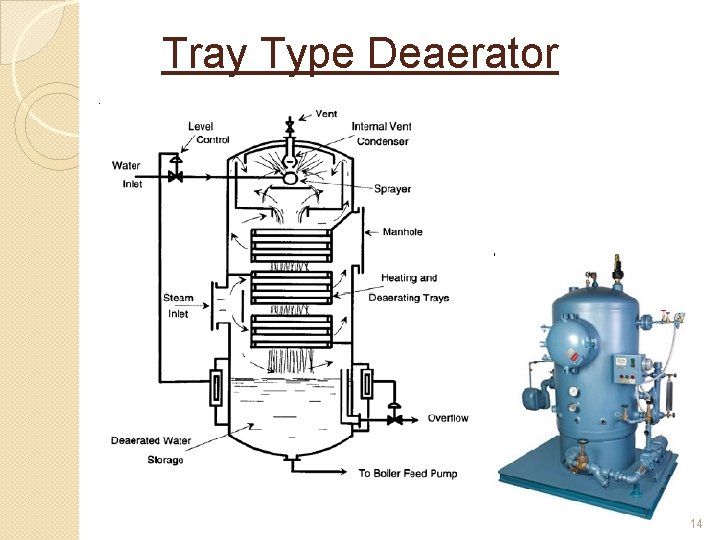
Tray Type Deaerator �The inlet water is broken up by trickling down over a series of trays. �The entering steam scrubs the water in the lower trays and heats the water in the upper tray section. �The released gases and remaining steam pass to the internal vent condenser, ◦ most of the steam is condensed and the gases are vented to atmosphere. �See next slide 13

Tray Type Deaerator 14

Chemical Deaeration of O 2 �Not all oxygen gas is removed by mechanical deaeration �The remainder is removed by chemical deaeration. �O 2 scavengers are chemicals that are used to remove the remaining O 2 �See the next slide 15

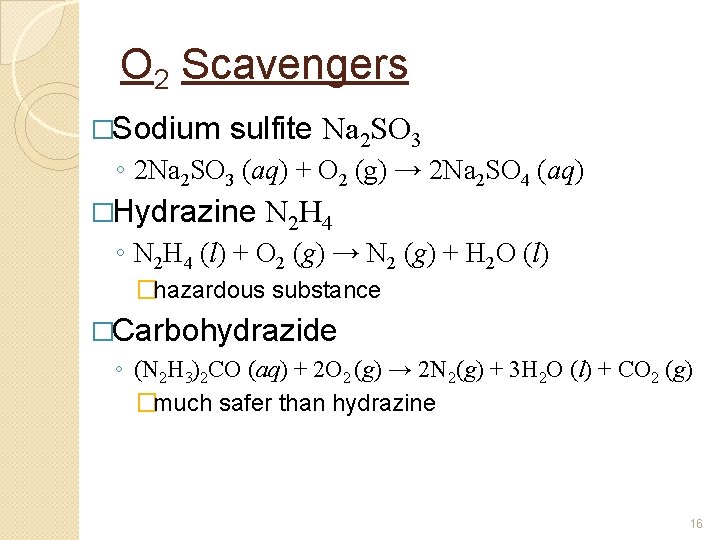
O 2 Scavengers �Sodium sulfite Na 2 SO 3 ◦ 2 Na 2 SO 3 (aq) + O 2 (g) → 2 Na 2 SO 4 (aq) �Hydrazine N 2 H 4 ◦ N 2 H 4 (l) + O 2 (g) → N 2 (g) + H 2 O (l) �hazardous substance �Carbohydrazide ◦ (N 2 H 3)2 CO (aq) + 2 O 2 (g) → 2 N 2(g) + 3 H 2 O (l) + CO 2 (g) �much safer than hydrazine 16

Internal Water treatment �Some impurities are present in the fresh makeup water. These concentrate in boiler water as more makeup water is added to replace steam losses. �Chemicals are added to the boiler to reduce problems caused by these impurities. �The proper level of chemical is insured by routinely testing boiler water for their presence.

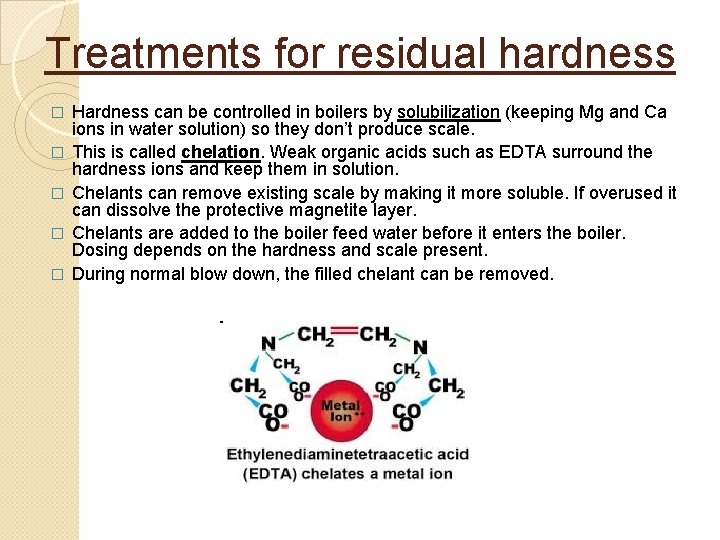
Treatments for residual hardness � � � Hardness can be controlled in boilers by solubilization (keeping Mg and Ca ions in water solution) so they don’t produce scale. This is called chelation. Weak organic acids such as EDTA surround the hardness ions and keep them in solution. Chelants can remove existing scale by making it more soluble. If overused it can dissolve the protective magnetite layer. Chelants are added to the boiler feed water before it enters the boiler. Dosing depends on the hardness and scale present. During normal blow down, the filled chelant can be removed.

Residual hardness can also be reduced by chemical precipitation as a non sticky sludge. The most insoluble form of calcium is calcium phosphate. This forms a non sticky sludge that can easily be removed during blow down. � Sodium phosphate may be added to boiler water to precipitate residual calcium hardness. � The sodium carbonate is soluble in boiler water. � The calcium phosphate produces a non sticky sludge. �

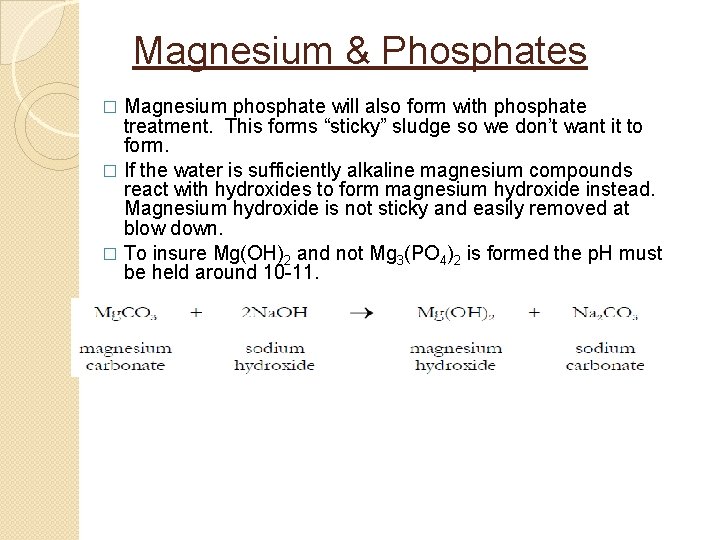
Magnesium & Phosphates Magnesium phosphate will also form with phosphate treatment. This forms “sticky” sludge so we don’t want it to form. � If the water is sufficiently alkaline magnesium compounds react with hydroxides to form magnesium hydroxide instead. Magnesium hydroxide is not sticky and easily removed at blow down. � To insure Mg(OH)2 and not Mg 3(PO 4)2 is formed the p. H must be held around 10 -11. �

Coordinated Phosphate p. H program � � � � � Different sodium phosphate compounds have a different impact on phosphate and p. H levels. MSP (monosodium phosphate) increases phosphate and decreases p. H by removing Na. OH � Na. H 2 PO 4 + Na. OH Na 2 HPO 4 + H 2 O DSP (disodium phosphate) increases phosphate and causes no change to p. H � Na 2 HPO 4 TSP (trisodium phosphate) increases phosphate and increases p. H by releasing Na. OH � Na 3 PO 4 + H 2 O Na 2 HPO 4 + Na. OH increases p. H no change of phosphate Blowdown decreases phosphate, lowers p. H 10 Ca(SO 4) (aq) + 2 Na. OH (aq)+ 6 Na 3(PO 4) (aq) Ca(OH)2. 3 Ca 3(PO 4)2 (s)+ 10 Na 2(SO 4) (aq) Calcium sulfate correct p. H-phosphate balance Calcium hydroxyapatite + Sodium sulfate The calcium hydroxyapatite (least soluble calcium compound) is a non sticky precipitate which remains suspended in the water and is easily removed during boiler blow down.
![TSP Increases p H Na 3 PO 4 TSP IncreasesPO 43 needed to TSP Increases p. H �Na 3 PO 4 (TSP) ◦ Increases[PO 43−] needed to](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-22.jpg)
TSP Increases p. H �Na 3 PO 4 (TSP) ◦ Increases[PO 43−] needed to remove Ca or Mg ◦ Increases p. H (use if the p. H is below the optimal range of [OH−] ) �This is due to the production of Na. OH in the reaction when TSP is added to the water 22
![DSP Maintains p H Na 2 HPO 4 DSP Increases PO 43 needed DSP Maintains p. H �Na 2 HPO 4 (DSP) ◦ Increases [PO 43−] needed](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-23.jpg)
DSP Maintains p. H �Na 2 HPO 4 (DSP) ◦ Increases [PO 43−] needed to remove Ca or Mg ◦ Maintains existing p. H (in the optimal range of [OH−] ) �no excess H+ or OH− produced 23
![MSP Decreases p H Na H 2 PO 4 MSP Increases PO 43 MSP Decreases p. H �Na. H 2 PO 4 (MSP) ◦ Increases [PO 43−]](https://slidetodoc.com/presentation_image_h2/3d837e8d412eb3f49acc8e807884a8f6/image-24.jpg)
MSP Decreases p. H �Na. H 2 PO 4 (MSP) ◦ Increases [PO 43−] needed to remove Ca or Mg ◦ Decreases p. H (if the p. H is above the optimal range of [OH−] ) �This is due to the production of H 3 PO 4 in the reaction when TSP is added to the water 24

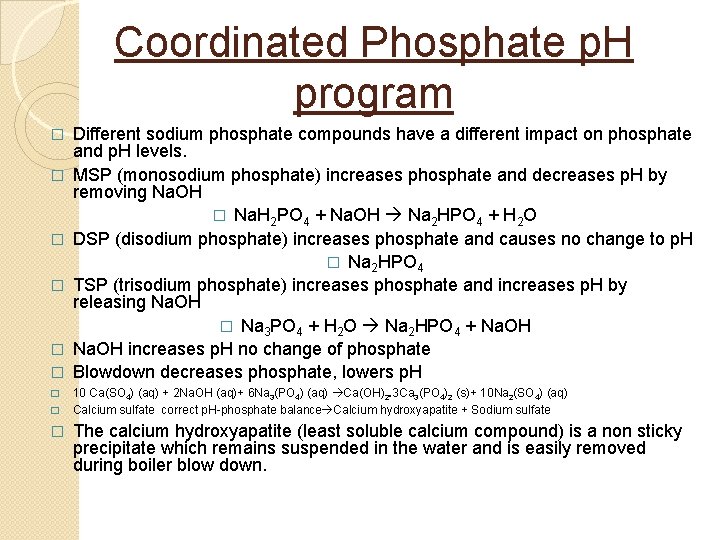
Sludge Conditioning Polymers Sludge is suspended solids in a boiler. These may come from the precipitation of dissolved solids such as hardness. � Polymers are sometimes used in combination with phosphates and chelants. � Boiler water polymers function as dispersants and condition sludge to not adhere to surfaces. This aids in their removal during blow down. � Polymers are long chain organic compounds that have negative charges their surface. These negatively charged groups attract positive charges on sludge and cause them coagulate. The larger particles settle to the bottom of the boiler and are removed during blow down. One such polymer unit (sulfonated polystyrene) is shown below. �

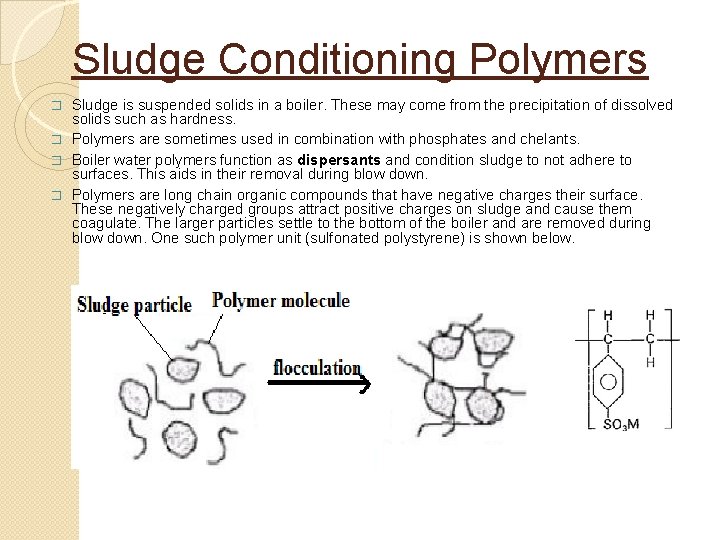
Typical Steam system Boiler/cooling/wastewater treatment 26