CH 2 MEASUREMENT I Using Measurements I II



















- Slides: 45

CH. 2 - MEASUREMENT I. Using Measurements I II III (p. 44 - 57)

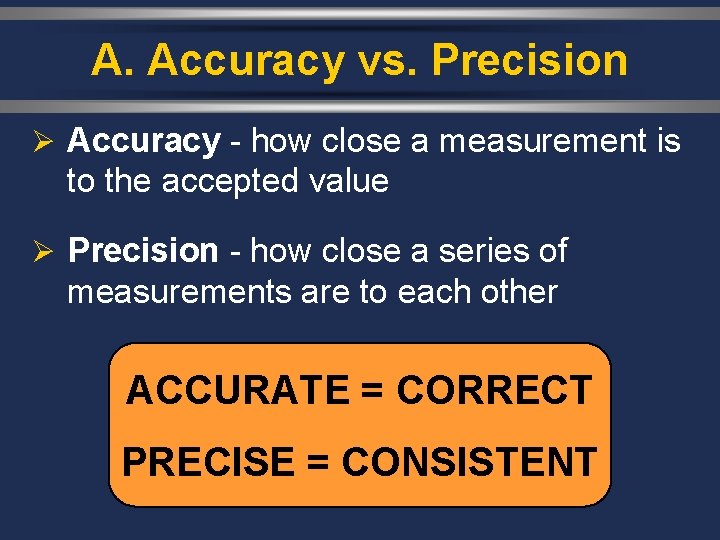
A. Accuracy vs. Precision Ø Accuracy - how close a measurement is to the accepted value Ø Precision - how close a series of measurements are to each other ACCURATE = CORRECT PRECISE = CONSISTENT

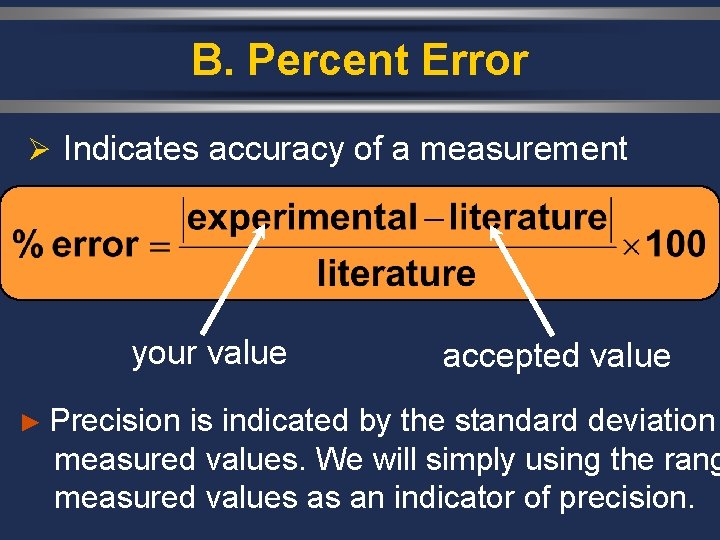
B. Percent Error Ø Indicates accuracy of a measurement your value accepted value ► Precision is indicated by the standard deviation measured values. We will simply using the rang measured values as an indicator of precision.

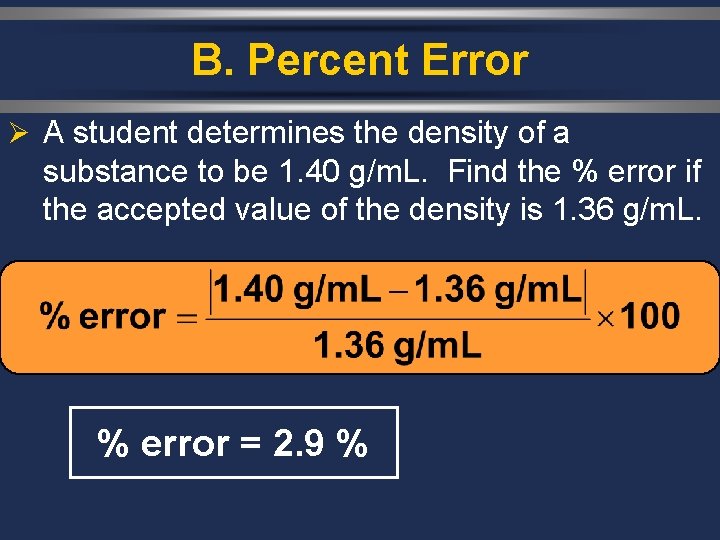
B. Percent Error Ø A student determines the density of a substance to be 1. 40 g/m. L. Find the % error if the accepted value of the density is 1. 36 g/m. L. % error = 2. 9 %

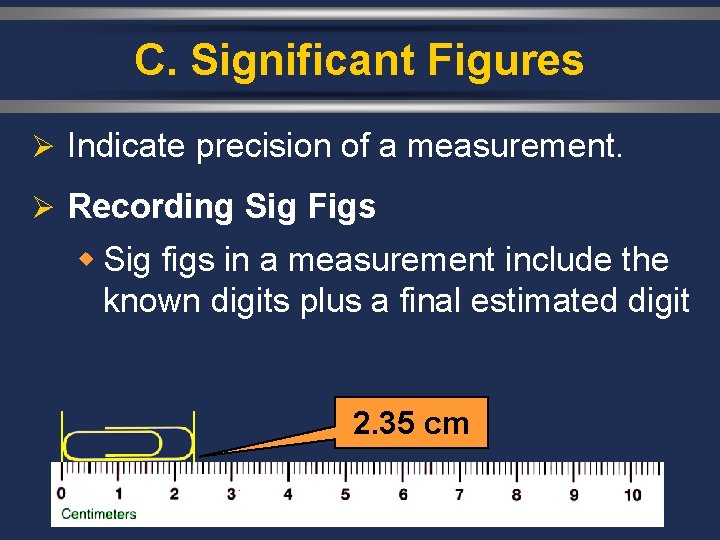
C. Significant Figures Ø Indicate precision of a measurement. Ø Recording Sig Figs w Sig figs in a measurement include the known digits plus a final estimated digit 2. 35 cm

Reading Instruments Ø Reading a meniscus on a graduated cylinder from eye level. Ø This graduated cylinder should be read to one decimal place. Ø 52. 8 m. L - The 8 is the estimated digit. Don’t forget units!

Try This: What is the volume on the graduated cylinder? 76. 0 m. L The 0 is the estimated digit

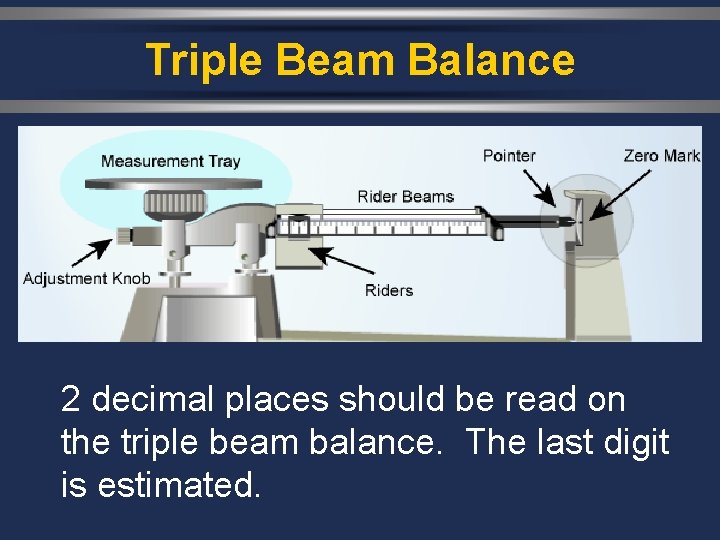
Triple Beam Balance 2 decimal places should be read on the triple beam balance. The last digit is estimated.

Random Error Ø Random errors are statistical fluctuations (in either direction) in the measured data due to the precision limitations of the measurement device. Ø Random errors usually result from the experimenter's inability to take the same measurement in exactly the same way to get exact the same number. Ø Can be minimized by taking repeated measurements.

Systematic Error Ø Systematic error (also called systematic bias) is consistent, Ø Ø repeatable error associated with faulty equipment or a flawed experiment design. These errors are usually caused by measuring instruments that are incorrectly calibrated or are used incorrectly. However, they can creep into your experiment from many sources, including: A worn out instrument. For example, a plastic tape measure becomes slightly stretched over the years, resulting in measurements that are slightly too high. An incorrectly calibrated or tared instrument, like a scale that doesn’t read zero when nothing is on it. A person consistently takes an incorrect measurement or rounds a measurement incorrectly. Not corrected by repeated measurements.

C. Significant Figures Ø Counting Sig Figs (Table 2 -5, p. 47) w Count all numbers EXCEPT: ² Leading zeros -- 0. 0025 ² Trailing zeros without a decimal point -- 2, 500

C. Significant Figures Counting Sig Fig Examples 1. 23. 50 4 sig figs 2. 402 3 sig figs 3. 5, 280 3 sig figs 4. 0. 080 2 sig figs

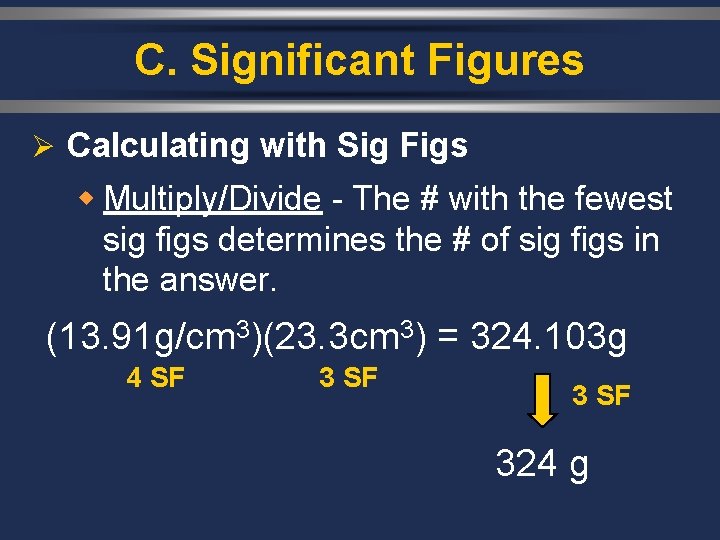
C. Significant Figures Ø Calculating with Sig Figs w Multiply/Divide - The # with the fewest sig figs determines the # of sig figs in the answer. (13. 91 g/cm 3)(23. 3 cm 3) = 324. 103 g 4 SF 324 g

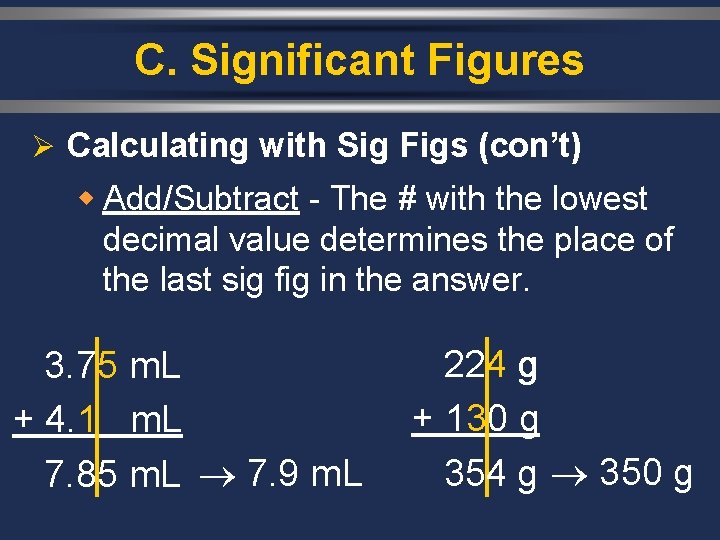
C. Significant Figures Ø Calculating with Sig Figs (con’t) w Add/Subtract - The # with the lowest decimal value determines the place of the last sig fig in the answer. 3. 75 m. L + 4. 1 m. L 7. 85 m. L 7. 9 m. L 224 g + 130 g 354 g 350 g

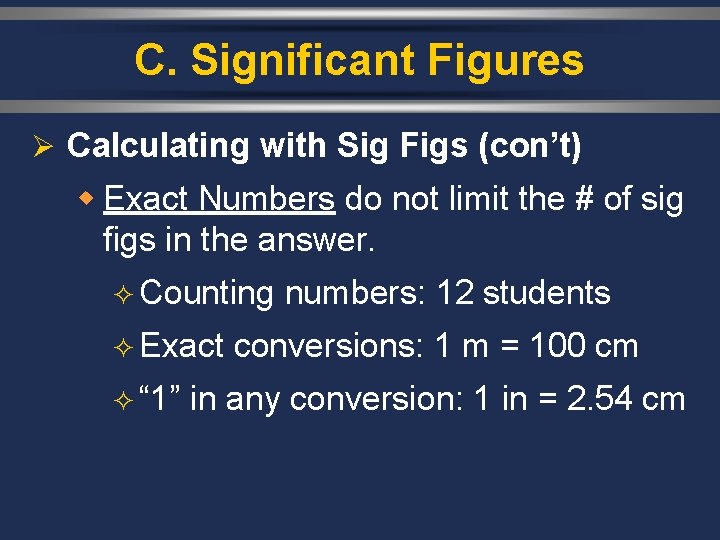
C. Significant Figures Ø Calculating with Sig Figs (con’t) w Exact Numbers do not limit the # of sig figs in the answer. ² Counting numbers: 12 students ² Exact conversions: 1 m = 100 cm ² “ 1” in any conversion: 1 in = 2. 54 cm

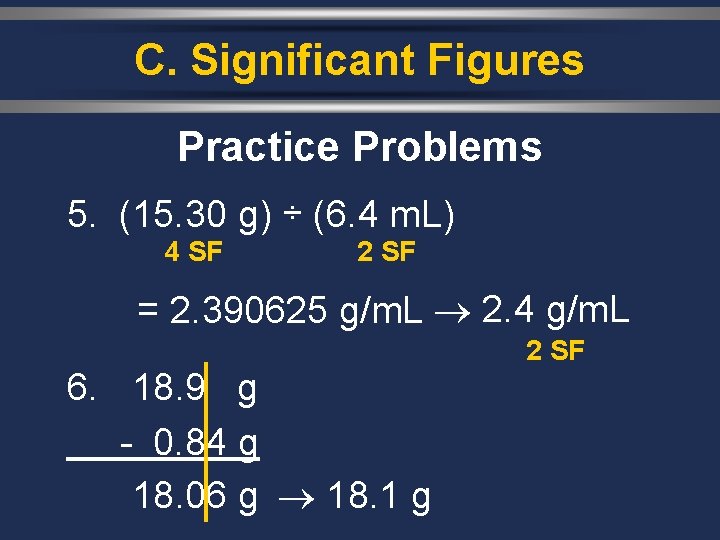
C. Significant Figures Practice Problems 5. (15. 30 g) ÷ (6. 4 m. L) 4 SF 2 SF = 2. 390625 g/m. L 2. 4 g/m. L 2 SF 6. 18. 9 g - 0. 84 g 18. 06 g 18. 1 g

D. Scientific Notation 65, 000 kg 6. 5 × 104 kg Ø Converting into Sci. Notation: w Move decimal until there’s 1 digit to its left. Places moved = exponent. w Large # (>1) positive exponent Small # (<1) negative exponent w Only include sig figs.

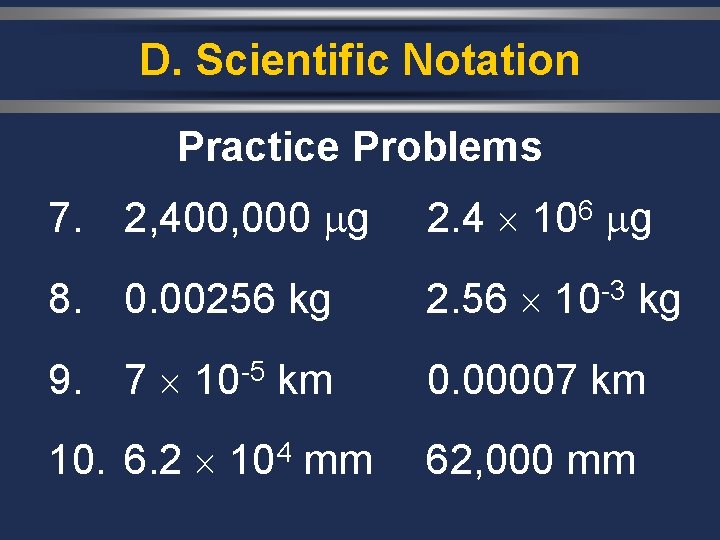
D. Scientific Notation Practice Problems 7. 2, 400, 000 g 6 2. 4 10 g 8. 0. 00256 kg -3 2. 56 10 kg 9. 7 10 -5 km 0. 00007 km 10. 6. 2 104 mm 62, 000 mm

Scientific Notation Practice 0. 000325 x 10 6 becomes 3. 25 x 102 in proper scientific notation This first number is not in proper scientific notation format - The number before the power of ten must be between 1 and 10. Since I had to move The decimal point 4 places to the right (the number got larger) I must reduce the exponent by 4. 127. 65 x 10 -8 becomes 1. 2765 x 10 -6 in proper scientific notation Here the number before the exponent was larger than 1 to 10 so I had to move the decimal point 2 places to the left (the

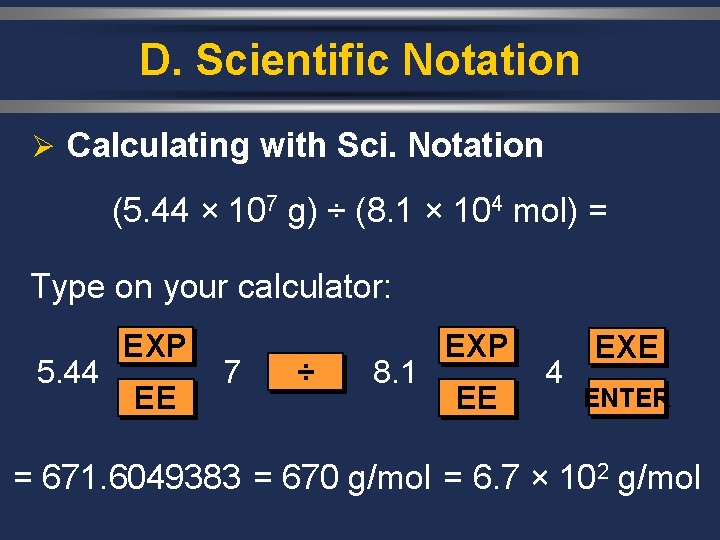
D. Scientific Notation Ø Calculating with Sci. Notation (5. 44 × 107 g) ÷ (8. 1 × 104 mol) = Type on your calculator: 5. 44 EXP EE 7 ÷ 8. 1 EXP EE 4 EXE ENTER = 671. 6049383 = 670 g/mol = 6. 7 × 102 g/mol

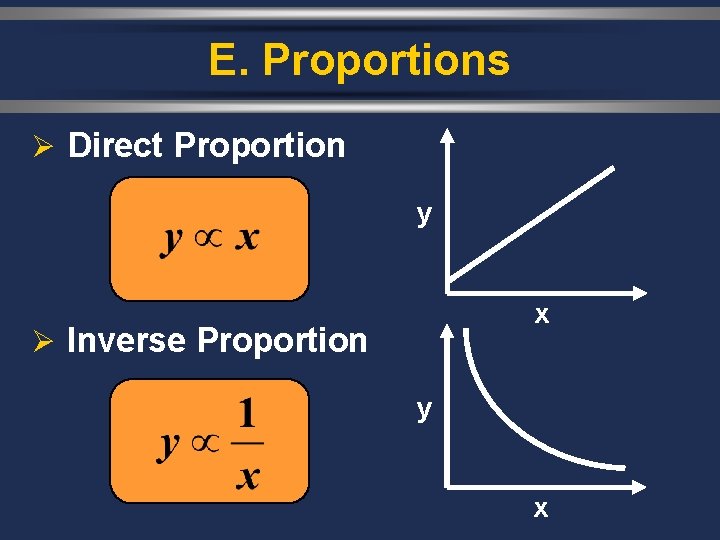
E. Proportions Ø Direct Proportion y x Ø Inverse Proportion y x

CH. 2 - MEASUREMENT II. Units of Measurement I II III (p. 33 - 39)

A. Number vs. Quantity Ø Quantity - number + unit UNITS MATTER!!

B. SI Units Quantity Symb Base Unit Abbre ol v. l Length meter m Mass m kilogram kg Time t second s Temp T kelvin K Amount n mole mol

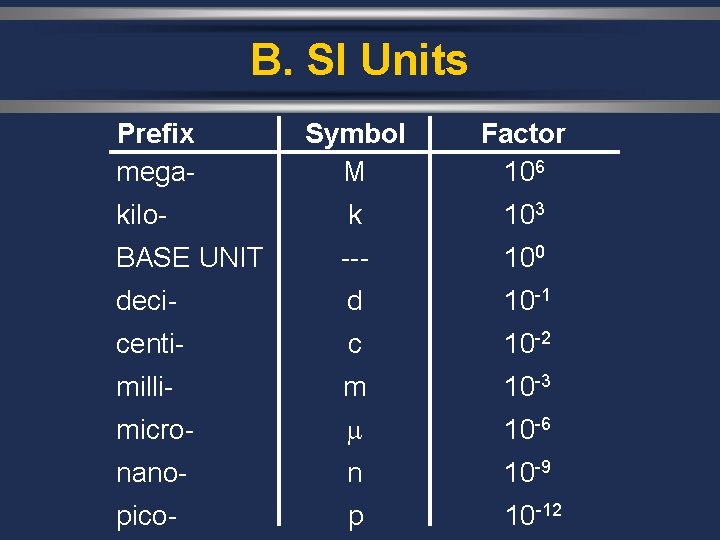
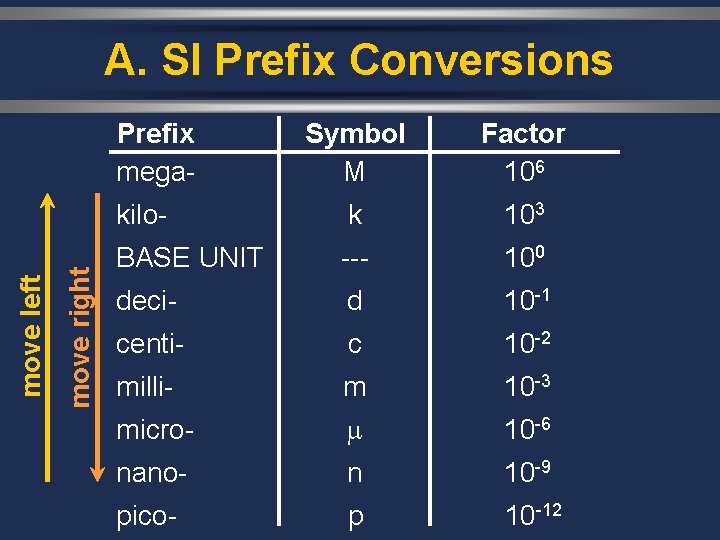
B. SI Units Prefix mega- Symbol M Factor 106 kilo- k 103 BASE UNIT --- 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12

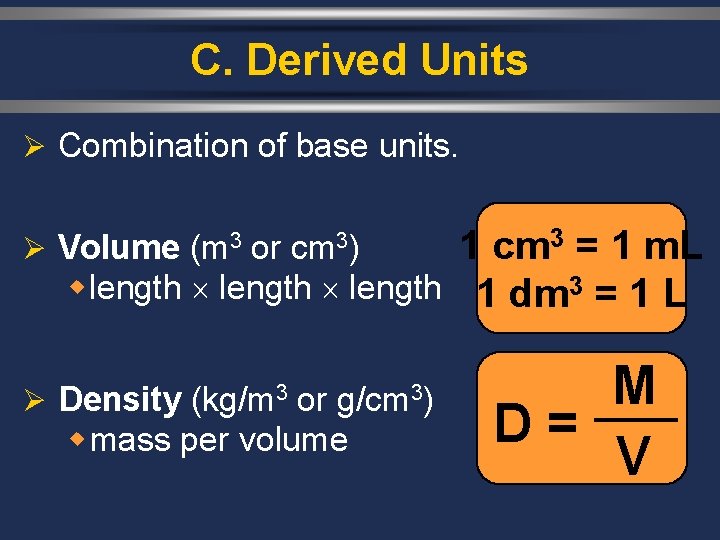
C. Derived Units Ø Combination of base units. 1 cm 3 = 1 m. L w length 1 dm 3 = 1 L Ø Volume (m 3 or cm 3) Ø Density (kg/m 3 or g/cm 3) w mass per volume M D = V

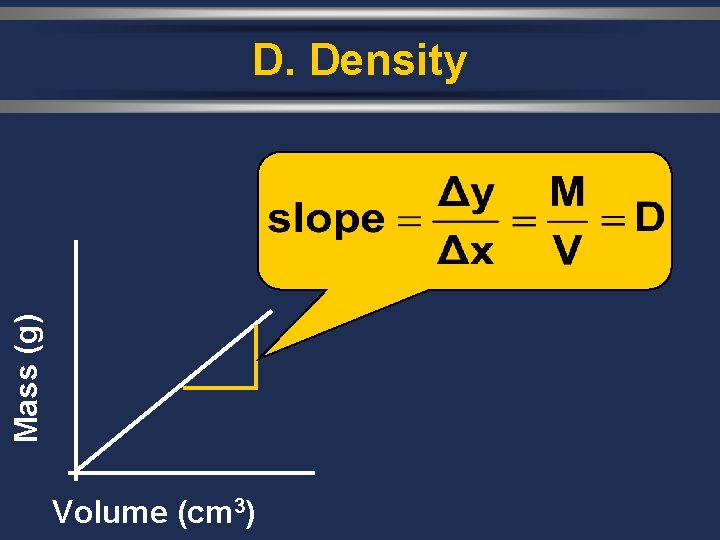
Mass (g) D. Density Volume (cm 3)

Problem-Solving Steps 1. Analyze 2. Plan 3. Compute 4. Evaluate

D. Density Ø An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M = ? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 200 g

D. Density Ø A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V = ? M = 25 g V = M D V = 25 g 0. 87 g/m. L V = 29 m. L

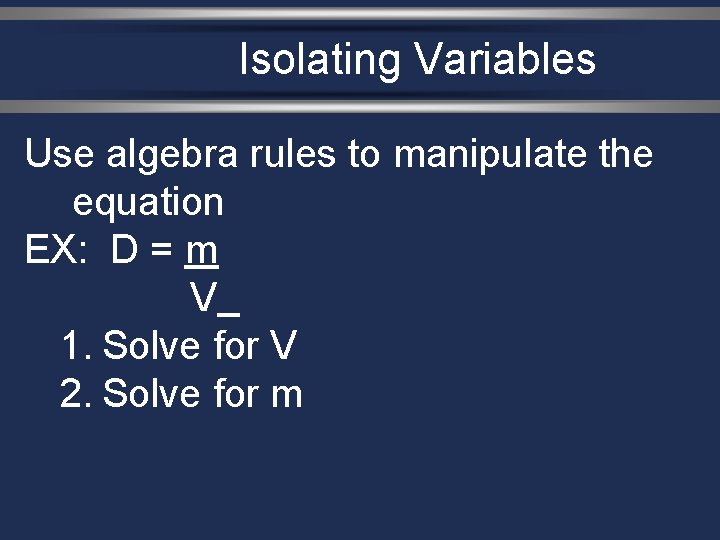
Isolating Variables Use algebra rules to manipulate the equation EX: D = m V 1. Solve for V 2. Solve for m

CH. 2 - MEASUREMENT III. Unit Conversions I II III (p. 40 - 42)

A. SI Prefix Conversions 1. Find the difference between the exponents of the two prefixes. 2. Move the decimal that many places. To the left or right?

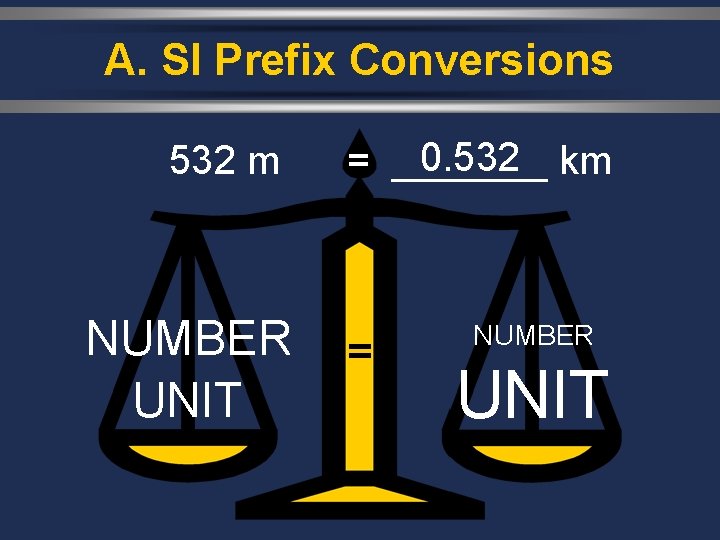
A. SI Prefix Conversions 0. 532 m = _______ km NUMBER UNIT = NUMBER UNIT

A. SI Prefix Conversions move right move left Prefix mega- Symbol M Factor 106 kilo- k 103 BASE UNIT --- 100 deci- d 10 -1 centi- c 10 -2 milli- m 10 -3 micro- 10 -6 nano- n 10 -9 pico- p 10 -12

A. SI Prefix Conversions 1) 20 cm = 0. 2 _______ m 32 2) 0. 032 L = _______ m. L 3) 45 m = 45000 _______ nm 0. 0805 4) 805 dm = _______ km

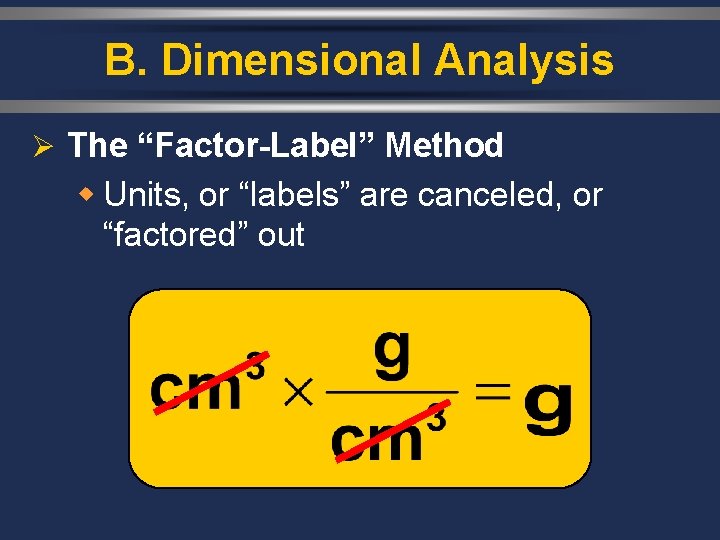
B. Dimensional Analysis Ø The “Factor-Label” Method w Units, or “labels” are canceled, or “factored” out

B. Dimensional Analysis Ø Steps: 1. Identify starting & ending units. 2. Line up conversion factors so units cancel. 3. Multiply all top numbers & divide by each bottom number. 4. Check units & answer.

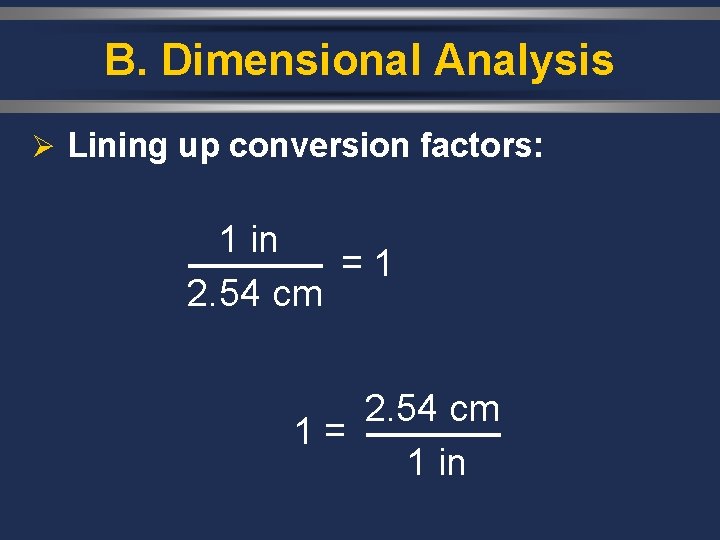
B. Dimensional Analysis Ø Lining up conversion factors: 1 in = 2. 54 cm = 1 2. 54 cm 1 in = 2. 54 cm 1 = 1 in

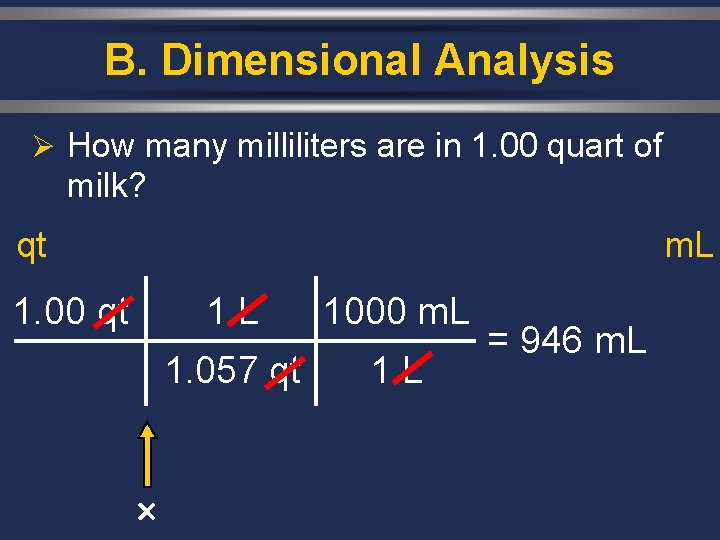
B. Dimensional Analysis Ø How many milliliters are in 1. 00 quart of milk? qt m. L 1. 00 qt 1 L 1000 m. L 1. 057 qt 1 L = 946 m. L

B. Dimensional Analysis Ø You have 1. 5 pounds of gold. Find its volume in cm 3 if the density of gold is 19. 3 g/cm 3. lb cm 3 1. 5 lb 1 kg 1000 g 1 cm 3 2. 2 lb 1 kg 19. 3 g = 35 cm 3

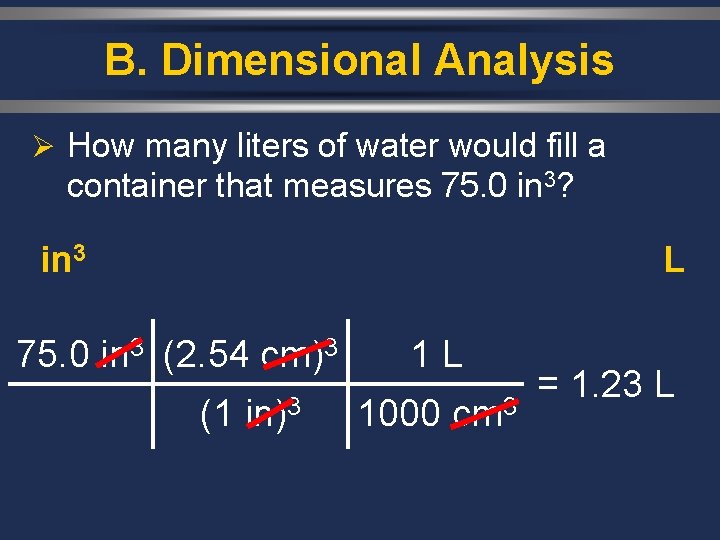
B. Dimensional Analysis Ø How many liters of water would fill a container that measures 75. 0 in 3? in 3 L 75. 0 in 3 (2. 54 cm)3 (1 in)3 1 L 1000 cm 3 = 1. 23 L

B. Dimensional Analysis 5) Your European hairdresser wants to cut your hair 8. 0 cm shorter. How many inches will he be cutting off? cm in 8. 0 cm 1 in 2. 54 cm = 3. 2 in

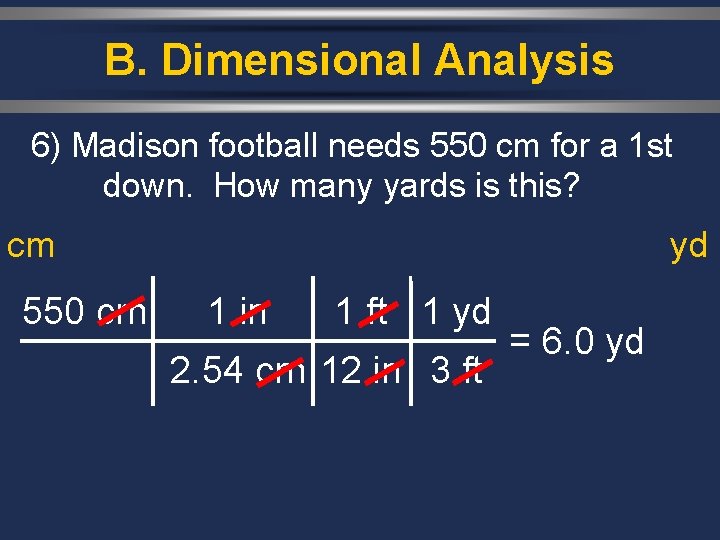
B. Dimensional Analysis 6) Madison football needs 550 cm for a 1 st down. How many yards is this? cm 550 cm yd 1 in 1 ft 1 yd 2. 54 cm 12 in 3 ft = 6. 0 yd

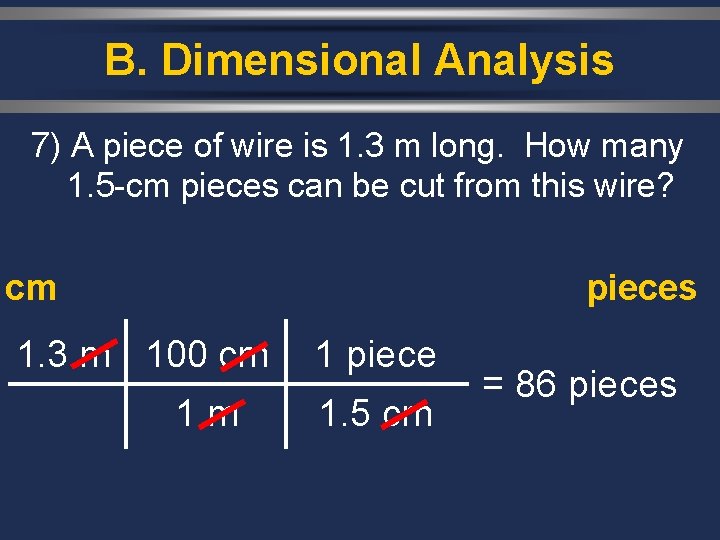
B. Dimensional Analysis 7) A piece of wire is 1. 3 m long. How many 1. 5 -cm pieces can be cut from this wire? cm pieces 1. 3 m 100 cm 1 piece 1. 5 cm = 86 pieces