Ch 11 Gases and Pressure 11 1 Pressure









- Slides: 11

Ch 11 Gases and Pressure 11. 1

Pressure and Force l Pressure – (P) the force per unit area on a surface l. Pressure = force area l P exerted by a gas depends on … l. Volume, ______, and # of molecules

Force l SI unit force is the ____ (N). l Newton – force that will increase the speed of a one kg mass by 1 meter per second for each second the force is applied l What is the force due to gravity? l How does this relate to what your ears do on an airplane?

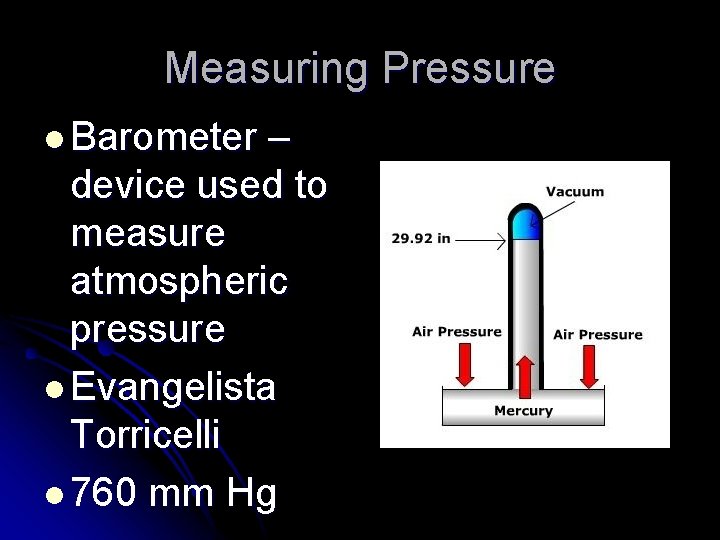
Measuring Pressure l Barometer – device used to measure atmospheric pressure l Evangelista Torricelli l 760 mm Hg

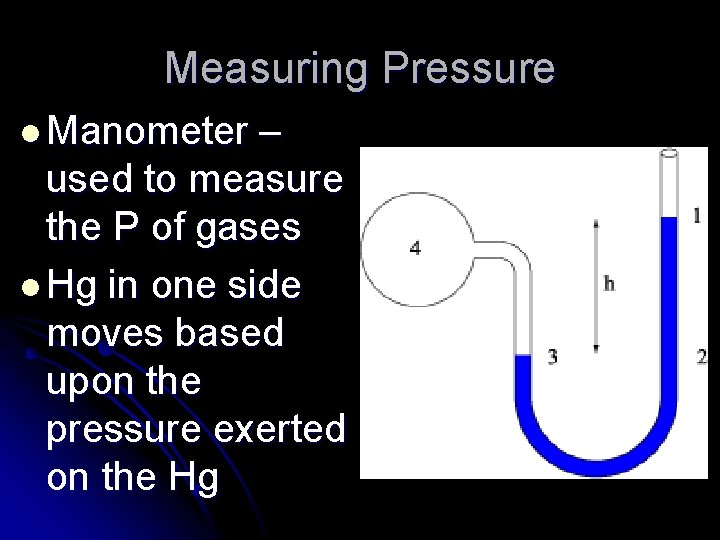
Measuring Pressure l Manometer – used to measure the P of gases l Hg in one side moves based upon the pressure exerted on the Hg

Units of Pressure l mm Hg – at 0 °C average atmospheric pressure is 760 mm Hg l 1 torr = mm Hg Unit named for __. l 1 atmosphere (atm) = 760 mm Hg l The SI unit for pressure is ____ l 101325 Pa = ______ k. Pa l 101. 325____ = 1____ = 760____

Pascal l Pascal (Pa) – the pressure exerted by a force of 1 newton (1 N) acting on an area of 1 square meter l Least used in science … PSI l 1 psi = 6892. 86 Pa l 1 atm = 14. 7 psi l STP = 1 atm & 0°C KNOW THIS

Calculations l Convert 0. 83 atm, the atm pressure in Denver, to mm Hg and k. Pa l Convert 1. 75 atm to k. Pa and mm Hg l Convert 72. 7 atm to Pa and torr.

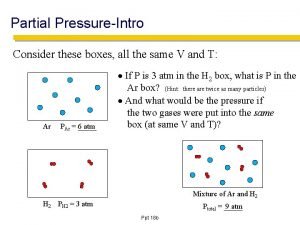
Dalton’s Law of Partial Pressure l Partial Pressure – the pressure of each gas in a mixture l Dalton’s Law of P. P. – the total P of a gas mix. is the + of the p. p. of the component gases l PT = P 1 + P 2 + P 3 + … l Why can u calc the total P of a mix by + the p. p of each?

Practice! l What is the total pressure of a container that has NH 3(g) exerting a pressure of 346 torr, N 2(g) exerting a pressure of 225 torr, and H 2 O (g) exerting a pressure of 55 torr?

Practice l What would be the total pressure of a container that is holding SO 2(g) with a pressure of. 385 atm, SO 3(g) with a pressure of 125 mm Hg, and N 2 O (g) with a pressure of 5. 67 psi? (Answer in atm. )
 Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Bernoulli's principle pipe flow
Bernoulli's principle pipe flow Oncotic vs osmotic pressure
Oncotic vs osmotic pressure Bevel of et tube
Bevel of et tube High pressure area
High pressure area Elmslie trillat
Elmslie trillat Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapleural pressure
Intrapleural pressure Oncotic pressure vs osmotic pressure
Oncotic pressure vs osmotic pressure How to find partial pressure from total pressure
How to find partial pressure from total pressure