CELL DEATH MECHANISMS Prof Dr Asuman SUNGUROLU Department


















![Cell death types Lorenzo Galluzzi, Ilio Vitale, […]Guido Kroemer Cell Death & Differentiation volume Cell death types Lorenzo Galluzzi, Ilio Vitale, […]Guido Kroemer Cell Death & Differentiation volume](https://slidetodoc.com/presentation_image_h2/a87317b772179834825d9117d5dcb98c/image-25.jpg)





- Slides: 31

CELL DEATH MECHANISMS Prof. Dr. Asuman SUNGUROĞLU Department of Medical Biology

The growth, development, and maintenance of multicellular organisms depend not only on the production of cells but also on mechanisms to destroy them. The maintenance of tissue size, for example, requires that cells die at the same rate as they are produced. During development, carefully orchestrated patterns of cell death help determine the size and shape of limbs and other tissues. Cells also die when they become damaged or infected, ensuring that they are removed before they threaten the health of the organism. Cell death is not a random process but occurs by a programmed sequence of molecular events, in which the cell systematically destroys itself from within and is then eaten by other cells, leaving no trace.

The knowledge of cell death mechanisms has evolved over the past decade and distinct cell death mechanisms have been described. Classically, cell death in mammalian cells was classified into three types of cell death: 1. Apoptosis (Type I cell death), 2. Autophagy-associated cell death (Type II cell death) 3. Necrosis (Type III cell death) 4. Entosis (Type IV cell death)

Animal cells that die in response to an acute insult, such as trauma or a lack of blood supply, usually do so by a process called cell necrosis. Necrotic cells swell and burst, spilling their contents over their neighbors and eliciting an inflammatory response In most cases, necrosis is likely to be caused by energy depletion, which leads to metabolic defects and loss of the ionic gradients that normally exist across the cell membrane. One form of necrosis, called necroptosis, is a form of programmed cell death that is triggered by a specific regulatory signal from other cells

Necroptosis Necrosis traditionally considered an accidental cell death that is not subject to cellular regulation. Extracellular ATP or death receptor activation rapidly induces receptor-interacting protein RIP 1/RIP 3 necrosome formation. Necrotic cell death results from the depletion of cytoplasmic ATP due to mitochondrial dysfunction. Necroptosis involves the loss of membrane integrity and the release of DAMPs, and is therefore associated with the development of inflammatory or anticancer immune responses

Apoptosis Eliminates Unwanted Cells In most cases, programmed cell death occurs by a process called apoptosis—from the Greek word meaning “falling off, ” as leaves from a tree. Cells dying by apoptosis undergo characteristic morphological changes. They shrink and condense, the cytoskeleton collapses, the nuclear envelope disassembles, and the nuclear chromatin condenses and breaks up into fragments The cell surface often bulges outward and, if the cell is large, it breaks up into membrane-enclosed fragments called apoptotic bodies. The surface of the cell or apoptotic bodies becomes chemically altered, so that a neighboring cell or a macrophage rapidly engulfs them

Apoptosis Pathway of cell death induced by a tightly regulated suicide program. *Controlled by specific genes. *Fragmentation of DNA. *Fragmentation of nucleus. *Shrinks of the cell memmbrane *Blebs form and apoptotic bodies are released. *Apoptotic bodies are phagocytosed

Apoptosis is largely modulated by the members of BCL-2 protein family including pro-apoptotic members (e. g. BAX, BAK, PUMA) and anti-apoptotic members (e. g. BCL-2 , BCL-XL, MCL-1 )

Apoptosis depends on an intracellular proteolytic cascade that is mediated by spesific proteases known as caspases *Initiator caspases are the effectors of the apoptotic process. *They normally exist as inactive and soluble monomers in the cytosol. *An apoptotic signal triggers the formation of the protein platforms that bring multiple initiator caspases together into large complexes. *These complexes, pairs of caspases associate to form dimers, resulting in protease activation (e. g caspase 8, 9)

Apoptosis is triggered by members of a family of specialized intracellular proteases, which cleave specific sequences in numerous proteins inside the cell, thereby bringing about the dramatic changes that lead to cell death and engulfment. These proteases have a cysteine at their active site and cleave their target proteins at specific aspartic acids; they are therefore called caspases. Caspases are synthesized in the cell as inactive precursors and are activated only during apoptosis. There are two major classes of apoptotic caspases: initiator caspases and executioner caspases.

CAD – caspase-activated DNase – Initial cleavage – 50, 000 base pairs – Ladder of DNA – 200 base pairs • ICAD – inhibitor of CAD Another target is a protein that normally holds a DNAdegrading endonuclease in an inactive form; its cleavage frees the endonuclease to cut up the DNA in the cell nucleus In healthy cells, the endonuclease CAD associates with its inhibitor, i. CAD. Activation of executioner caspases in the cell leads to cleavage of i. CAD, which unleashes the nuclease. Activated CAD cuts the chromosomal DNA between nucleosomes, resulting in the production of DNA fragments that form a ladder pattern upon gel electrophoresis.

Characteristic of the Apoptotic cells: 1. Chromosomal DNA cleaved into fragments 2. Change in the plasma membrane – phosphatidylserine exposure at the outer plasma membrane 3. Loss of electrical potential across the inner membrane of the mitochondria 4. Release of cytochrome c from the intermembrane space of the mitochondria to the cytosol

CASPASES Caspases involved in inflammation caspases 1 , 4, 5 Caspases involved in apoptosis Initiator caspases Executioner caspases 2, 8, 9, 10 caspases 3, 6, 7

Extracellular signal proteins binding to cell-surface death receptors trigger the extrinsic pathway of apoptosis. Death receptors are transmembrane proteins containing an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular death domain, which is required for the receptors to activate the apoptotic program. The receptors are homotrimers and belong to the tumor necrosis factor (TNF) receptor family, which includes a receptor for TNF itself and the Fas death receptor. The ligands that activate the death receptors are also homotrimers; they are structurally related to one another and belong to the TNF family of signal proteins.

A well-understood example of how death receptors trigger the extrinsic pathway of apoptosis is the activation of Fas on the surface of a target cell by Fas ligand on the surface of a killer (cytotoxic) lymphocyte. the death domains on the cytosolic tails of the Fas death receptors bind intracellular adaptor proteins when activated by the binding of Fas ligand which is binds to initiator caspases (primarily caspase-8) and forms a death-inducing signaling complex (DISC). Once dimerized and activated in the DISC, the initiator caspases cleave their partners and then activate downstream executioner caspases to induce apoptosis.

The extrinsic pathway of apoptosis activated through Fas death receptors. Trimeric Fas ligands on the surface of a killer lymphocyte interact with trimeric Fas receptors on the surface of the target cell, leading to clustering of several ligand-bound receptor trimers. Receptor clustering activates death domains on the receptor tails, which interact with similar domains on the adaptor protein FADD (FADD stands for Fas-associated death domain). Each FADD protein then recruits an initiator caspase (caspase-8) via a death effector domain on both FADD and the caspase, forming a death-inducing signaling complex (DISC). Within the DISC, two adjacent initiator caspases interact and cleave one another to form an activated protease dimer, which then cleaves itself in the region linking the protease to the death effector domain. This stabilizes and releases the active caspase dimer into the cytosol, where it activates executioner caspases by cleaving them.

Intrinsic Pathway of Apoptosis is regulated by Bcl 2 Proteins The intrinsic pathway of apoptosis is tightly regulated to ensure that cells kill themselves only when it is appropriate. A major class of intracellular regulators of the intrinsic pathway is the Bcl 2 family of proteins It has been conserved in evolution from worms to humans. Human Bcl 2 protein can suppress apoptosis when expressed in the worm C. elegans(CED 9)

When an apoptotic stimulus triggers the intrinsic pathway, the pro-apoptotic effector Bcl 2 family proteins(e. g Bax, Bak) become activated and aggregate to form oligomers in the mitochondrial outer membrane, inducing the release of cytochrome c and other intermembrane proteins In mammalian cells, Bax and Bak are the main effector of Apoptotic cell death Bak is bound to the mitochondrial outer membrane even in the absence of an apoptotic signal. Bax is mainly located in the cytosol and translocates to the mitochondria only after an apoptotic stimulus

Type II Programmed cell death: Autophagy-associated cell death (ACD) is another form of programmed cell death process that is triggered by self-digestion of cellular contents through autophagy. Autophagy was initially described as a homeostatic strategy to remove protein aggregates and dysfunctional organelles e. g mitochondria or endoplasmic reticulum and, thus, to provide an alternative source of energy when nutrients are scarce. Autophagic processes are involved in a wide range of normal physiological situations (such as mammalian development, metabolism, immunity and aging)


Autophagy: cellular selfcatabolic process "eating oneself” There are several different classifications in Autophagy Macroautophagy - sequestration of cytoplasmic substrates including organelles Microautophagy - lysosomal invagination and ingestion of minor portions of the cytosol Chaperone-mediated Autophagy - involves recognition of specific substrates by Hsc 70 (heat shock cognate 70) machinery to remove abnormal cytoplasmic organelles and other components

Macroautophagy is initiated by the formation of autophagosome means a double-membrane vesicle containing cytoplasmic materials. The outer membrane of the autophagosome subsequently fuses with the lysosomal membranes After that it forms the acid single membrane enclosed vacuoles, termed “autolysosomes” or autophagolysosomes. The sequestered material is then hydrolyzed by the lysosomal enzymes (acid hyrdrolases) and released in the cytoplasm following degradation of the autolysosome membrane


Autophagy is strongly regulated by a family of “autophagy-related” proteins named ATG proteins AUTOPHAGY-RELATED GENE (Atg gene) Autophagy is strongly regulated by a family of “autophagy-related” proteins named ATG proteins Atg 5 : Atg 5 -Atg 12 Biogenesis of autophagic vesicles (autophagosome) Beclin 1 (Atg 6):a Bcl-2 interacting protein forms complex with PI 3 K to regulate autophagy. LC 3 (Atg 8):Normally spread throughout the cytoplasm. But, specifically concentrated on autophagosomes
![Cell death types Lorenzo Galluzzi Ilio Vitale Guido Kroemer Cell Death Differentiation volume Cell death types Lorenzo Galluzzi, Ilio Vitale, […]Guido Kroemer Cell Death & Differentiation volume](https://slidetodoc.com/presentation_image_h2/a87317b772179834825d9117d5dcb98c/image-25.jpg)
Cell death types Lorenzo Galluzzi, Ilio Vitale, […]Guido Kroemer Cell Death & Differentiation volume 25, pages 486– 541(2018)

Atypical Cell Death This section is not as relevant as "typical" cell death sections and is based upon the 2018 recommendations. A variety of historic and specialised terminologies that have arisen within the scientific literature. Accidental cell death (ACD). Virtually instantaneous and uncontrollable form of cell death corresponding to the physical disassembly o the plasma membrane caused by extreme physical, chemical, or mechanical cues. Programmed cell death (PCD). Particular form of RCD that occurs in strictly physiological scenarios, i. e. , it does not relate to perturbations of homeostasis and hence does not occur in the context of failing adaptation to stress. Anoikis. Specific variant of intrinsic apoptosis initiated by the loss of integrin-dependent anchorage. Autophagy-dependent cell death. A form of RCD that mechanistically depends on the autophagic machinery (or components thereof). Autosis. A specific instance of autophagy-dependent cell death that critically relies on the plasma membrane Na+/K+-ATPase. Cell death. Irreversible degeneration of vital cellular functions (notably ATP production and preservation of redox homeostasis) culminating in the loss of cellular integrity (permanent plasma membrane permeabilization or cellular fragmentation). Cellular senescence. Irreversible loss of proliferative potential associated with specific morphological and biochemical features, including the senescence-associated secretory phenotype (SASP). Cellular senescence does not constitute a form of RCD. Efferocytosis. Mechanism whereby dead cells and fragments thereof are taken up by phagocytes and disposed. Entotic cell death. A type of RCD that originates from actomyosin-dependent cell-in-cell internalization (entosis) and is executed by lysosomes.

Extrinsic apoptosis. Specific variant of RCD initiated by perturbations of the extracellular microenvironment detected by plasma membrane receptors, propagated by CASP 8 and precipitated by executioner caspases, mainly CASP 3. Ferroptosis. A form of RCD initiated by oxidative perturbations of the intracellular microenvironment that is under constitutive control by GPX 4 and can be inhibited by iron chelators and lipophilic antioxidants. Immunogenic cell death. A form of RCD that is sufficient to activate an adaptive immune response in immunocompetent hosts. Intrinsic apoptosis. Type of RCD initiated by perturbations of the extracellular or intracellular microenvironment, demarcated by MOMP, and precipitated by executioner caspases, mainly CASP 3. Lysosome-dependent cell death. A type of RCD demarcated by primary LMP and precipitated by cathepsins, with optional involvement of MOMP and caspases. Mitochondrial permeability transition (MPT)-driven necrosis. Specific form of RCD triggered by perturbations of the intracellular microenvironment and relying on CYPD. Mitotic catastrophe. Oncosuppressive mechanism for the control of mitosis-incompetent cells by RCD or cellular senescence. Per se, mitotic catastrophe does not constitute a form or RCD. Mitotic death. Specific variant of RCD (most often, intrinsic apoptosis) driven by mitotic catastrophe.

Necroptosis. A modality of RCD triggered by perturbations of extracellular or intracellular homeostasis that critically depends on MLKL, RIPK 3, and (at least in some settings) on the kinase activity of RIPK 1. NETotic cell death. A ROS-dependent modality of RCD restricted to cells of hematopoietic derivation and associated with NET extrusion. Parthanatos. A modality of RCD initiated by PARP 1 hyperactivation and precipitated by the consequent bioenergetic catastrophe coupled to AIF -dependent and MIF-dependent DNA degradation. Pyroptosis. A type of RCD that critically depends on the formation of plasma membrane pores by members of the gasdermin protein family, often (but not always) as a consequence of inflammatory caspase activation. Regulated cell death (RCD). Form of cell death that results from the activation of one or more signal transduction modules, and hence can be pharmacologically or genetically modulated (at least kinetically and to some extent).


Entosis(Tip IV Cell Death) It is well known that apoptotic cells are commonly engulfed byprofessional phagocytes or by non-professional neighboring cells. However, living cells may be also engulfed by other cells, which induce an atypical cell death process This lethal mechanism is termed entosis (from the Greek word entos, which means inside, into, or within). Entosis provides an interesting case of xenophagy means intracellular destruction of foreign organisms and was first discovered in human tumors. Interactions between cancer cells initiate the formation of adherent junctions containing E-cadherins and b-catenins allowing a cellular invasion which is characterized by “cell-in -cell” cytological features.

Type IV cell death (Entosis, cellular cannibalism) Entosis - form of ‘cellular cannibalism’ in lymphoblasts from patients with Huntington's disease. Engulfed cells could inversely induce host cell death. Natural killer (NK) cells could be internalized within tumor cells, leading to either tumor cell death or self destruction within target cells. Review Article Entosis: The emerging face of non-cell autonomous type IV programmed death b i o m e d i c a l j o u r n a l 4 0 ( 2 0 1 7 ) 1 3 3 e 1 4 0
 Asuman reyhan kurt
Asuman reyhan kurt Gizem memiç türkiye güzeli
Gizem memiç türkiye güzeli Asuman doğaç
Asuman doğaç Aglutinini
Aglutinini Asuman doğaç
Asuman doğaç Asuman gölgeli
Asuman gölgeli Posteriör
Posteriör Asuman sunguroğlu
Asuman sunguroğlu Asuman reyhan kurt
Asuman reyhan kurt Somatic death vs molecular death
Somatic death vs molecular death Cell city analogy
Cell city analogy Denuding tower
Denuding tower Prokaryotic cell vs eukaryotic cell
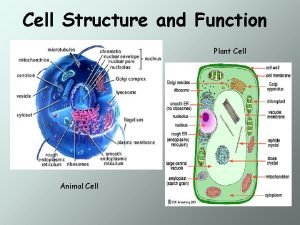
Prokaryotic cell vs eukaryotic cell Linear chromosomes in eukaryotes
Linear chromosomes in eukaryotes Plant vs animal cells organelles
Plant vs animal cells organelles Define concentration cells
Define concentration cells Dry cell vs wet cell
Dry cell vs wet cell Venn diagram animal and plant cell
Venn diagram animal and plant cell Function of cells
Function of cells Plant cell structure
Plant cell structure Smooth endoplasmic reticulum function simple
Smooth endoplasmic reticulum function simple Cell wall cell membrane
Cell wall cell membrane Cell strain
Cell strain Finite and continuous cell lines
Finite and continuous cell lines Cell city project
Cell city project Primary voltaic cell
Primary voltaic cell Whats the difference between plant and animal cells
Whats the difference between plant and animal cells Cell-cell junction
Cell-cell junction Cell-cell junction
Cell-cell junction Which organelle prepares proteins for specific jobs
Which organelle prepares proteins for specific jobs Section 10-2 cell division
Section 10-2 cell division Life
Life