CEE 437 Lecture 2 Minerals Thomas Doe Topics









- Slides: 22

CEE 437 Lecture 2 Minerals Thomas Doe

Topics • • • Mineral Definition Rock Forming Minerals Physical Proprieties of Minerals Mineral Identification Mineral Lab

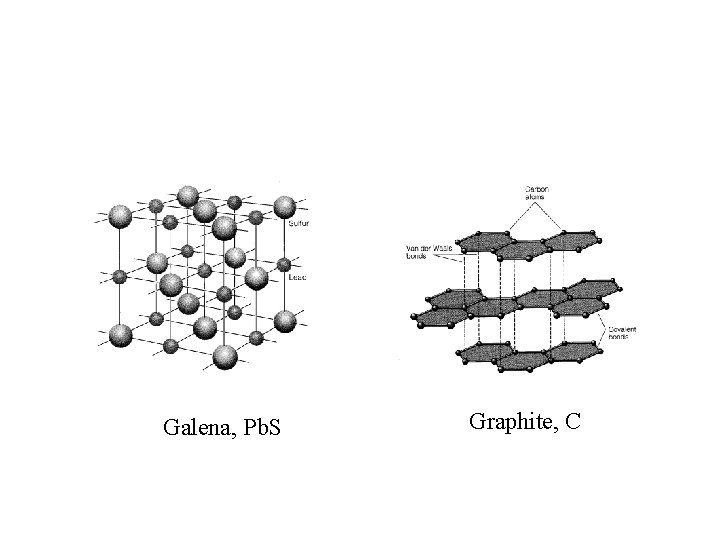
Mineral Definition • Naturally occurring material with unique combination of chemical composition and crystalline structure • Natural non-minerals — glasses, coal, amorphous silica • Pseudomorphs: diamond: graphite

Galena, Pb. S Graphite, C

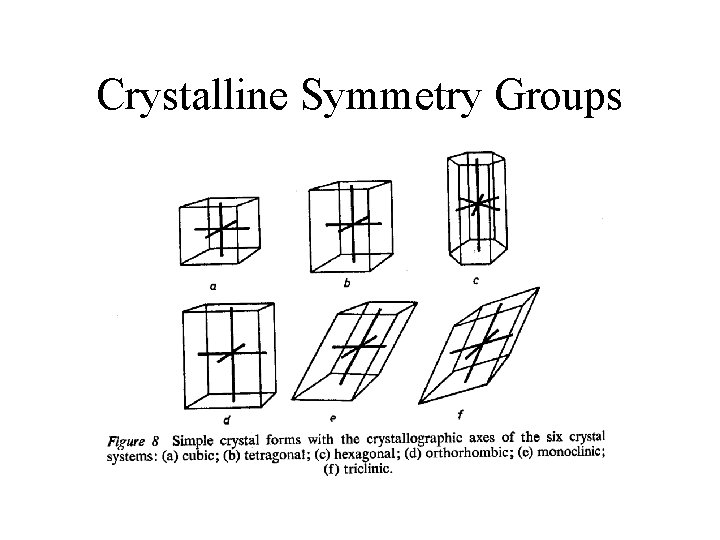
Crystalline Symmetry Groups

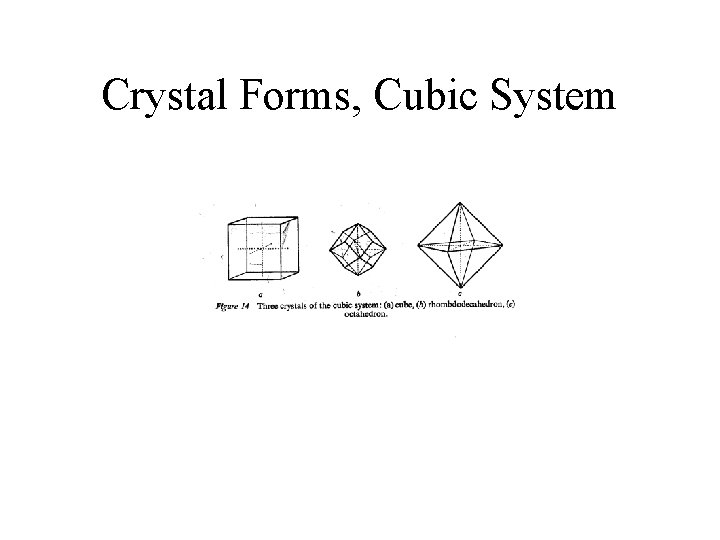
Crystal Forms, Cubic System

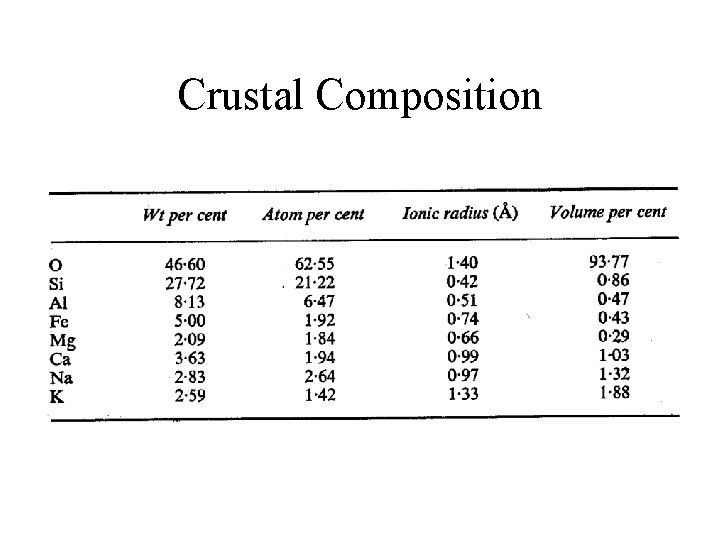
Crustal Composition

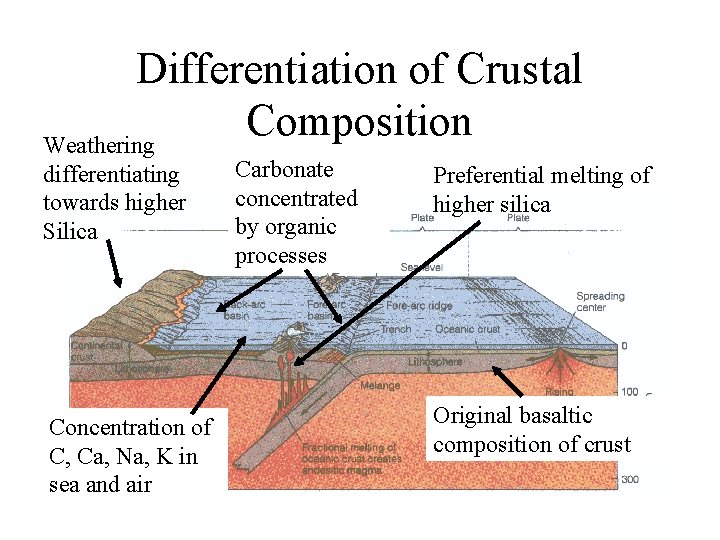
Differentiation of Crustal Composition Weathering differentiating towards higher Silica Concentration of C, Ca, Na, K in sea and air Carbonate concentrated by organic processes Preferential melting of higher silica Original basaltic composition of crust

Mineral Differentiation • Plate tectonics – selective melting, selective recrytallization – differentiation by density • Weathering and erosion

Elemental Fates • Silicon tends to concentrate in crust — quartz is very long lived • Aluminum — transforms from feldspars to clays • Mica — transform to clays • Fe-Mg-Ca-Na-K concentrate in some clays and micas, concentrate in oceans in biosphere

Rock Forming Minerals • Composition of Crust – Dominantly O, Si, Fe, Mg, Ca, Na, K – Near surface importance of bio-processes – Silicates from inorganic processes – Carbonates mainly from shell-forming organisms

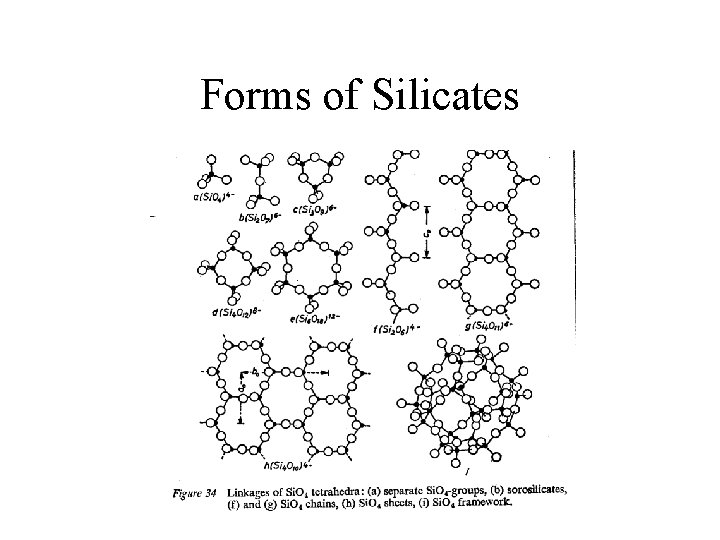
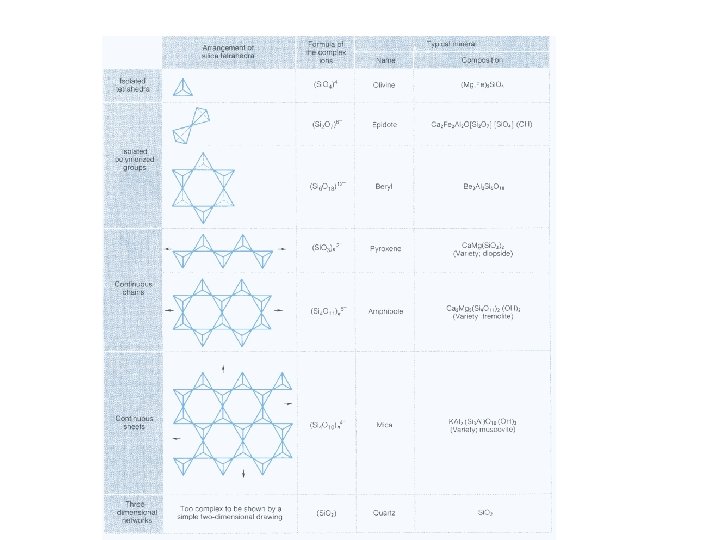
Major Silicate Groups • Silicon Tetrahedron – separate tetraheadra — olivine – single chains — pyroxene – double chains — amphibole – sheet silicates — micas and clays – framework silicates — feldspars (with Al substitution), quartz as pure silica

Forms of Silicates


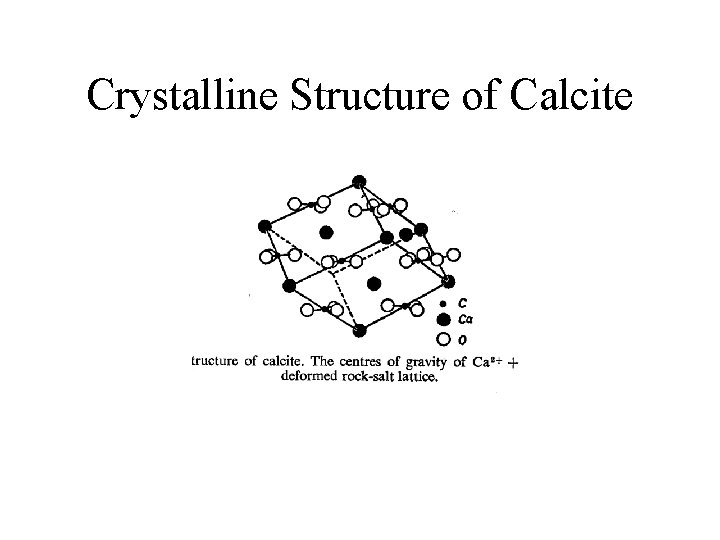
Crystalline Structure of Calcite

Physical Properties • • • Density (Gravity) Electrical Conductivity (Resisitivity) Thermal Expansion Strength Elasticity (Mechanical properties, – Seismic/Acoustic Velocity • Rheology (Plasticity, Viscosity)

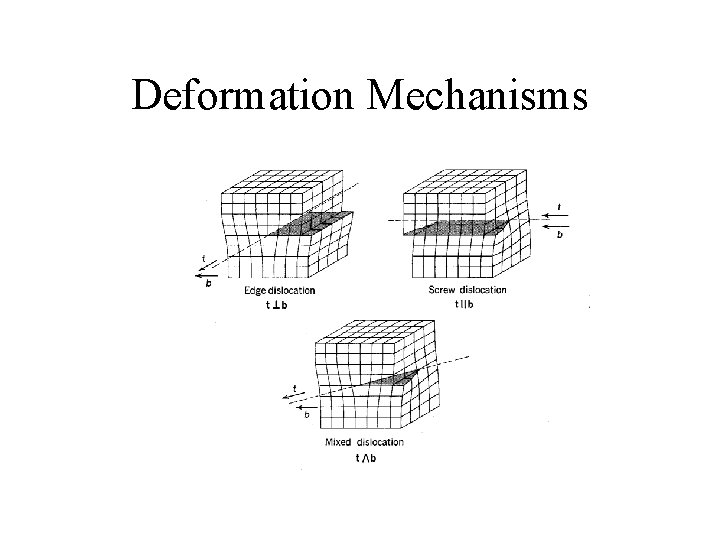
Deformation Mechanisms

Effects on Physical Properties • Anisotropy – Properties differ by direction • Heterogeneity – Properties vary by location • Mineral properties may have strong anisotropy when crystals are aligned • Heterogeneity may have strong mechanical effects when different minerals have different deformation properties

Clay Viewed from Electron Microscope

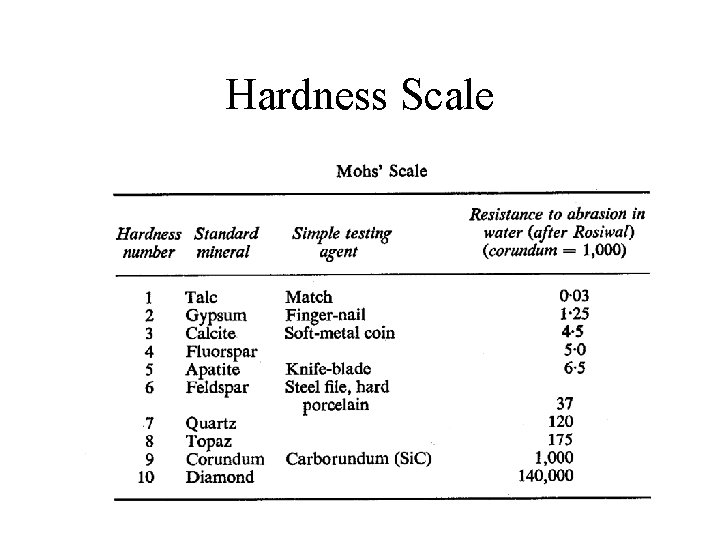
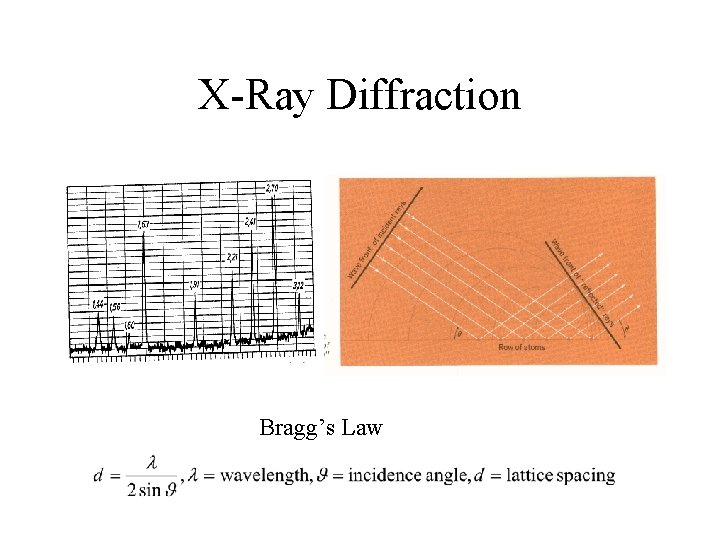
Mineral Identification • Density • Hardness • Color, luster (metallic, non-metalic, semimetallic) • Crystalline habit • Cleavage • Mineral chemistry, x-ray diffraction

Hardness Scale

X-Ray Diffraction Bragg’s Law