Calculating H using molar heats of formation Chem




- Slides: 11

Calculating ΔH using molar heats of formation Chem 12

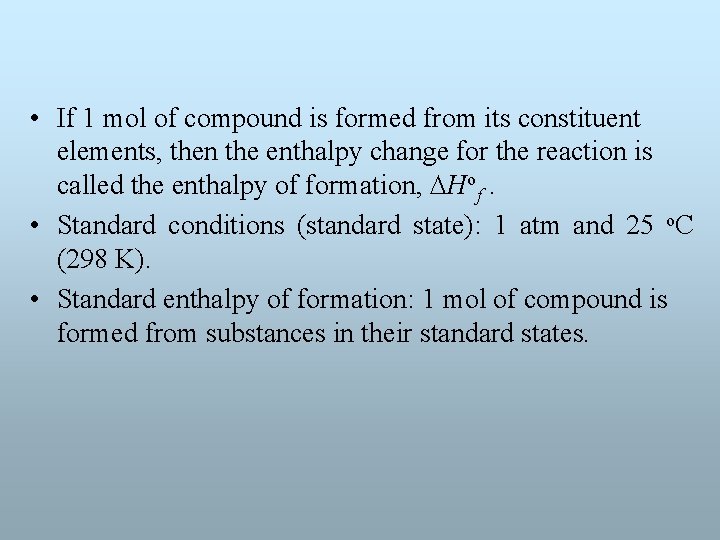
• If 1 mol of compound is formed from its constituent elements, then the enthalpy change for the reaction is called the enthalpy of formation, Hof. • Standard conditions (standard state): 1 atm and 25 o. C (298 K). • Standard enthalpy of formation: 1 mol of compound is formed from substances in their standard states.

Examples – write formation reactions for each: *Remember the compounds are formed directly from their elements. 1. H 2 SO 4 2. NH 4 Cl

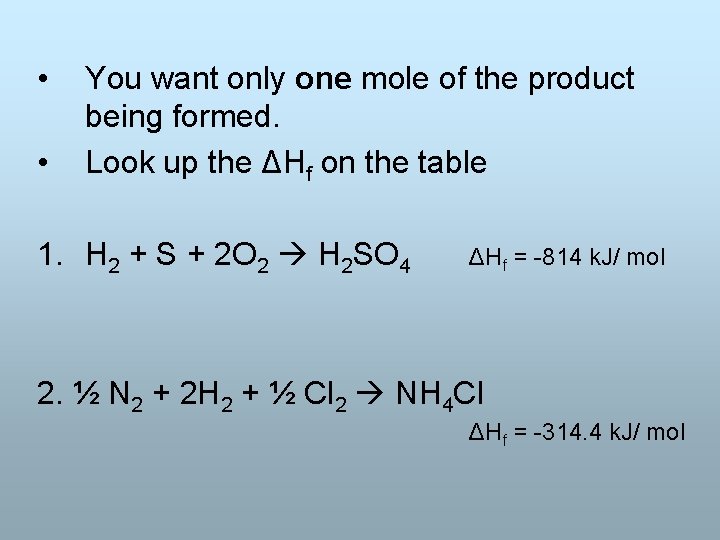
• • You want only one mole of the product being formed. Look up the ΔHf on the table 1. H 2 + S + 2 O 2 H 2 SO 4 ΔHf = -814 k. J/ mol 2. ½ N 2 + 2 H 2 + ½ Cl 2 NH 4 Cl ΔHf = -314. 4 k. J/ mol

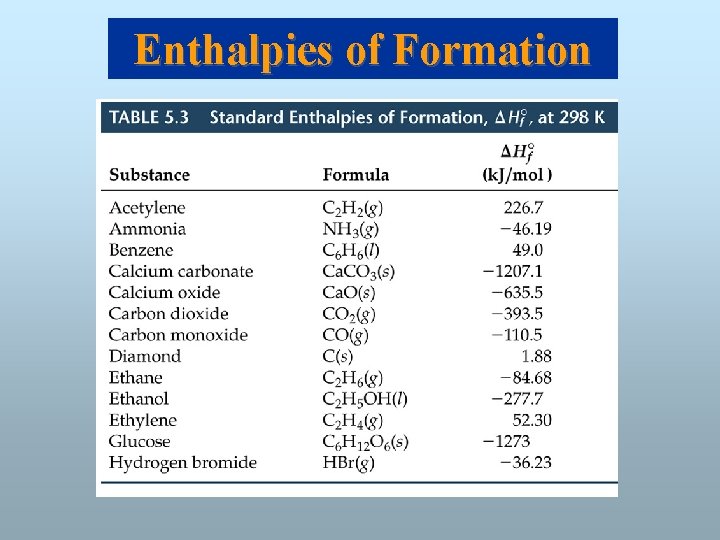
Enthalpies of Formation

Using Enthalpies of Formation for Calculating Enthalpies of Reaction • For a reaction

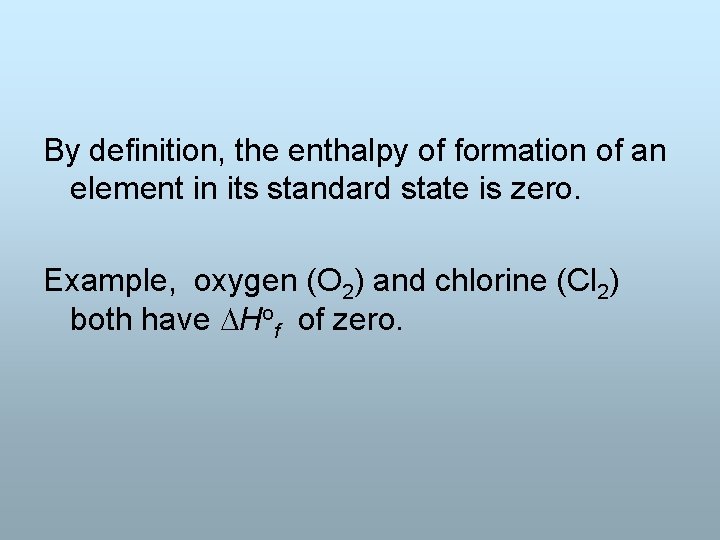
By definition, the enthalpy of formation of an element in its standard state is zero. Example, oxygen (O 2) and chlorine (Cl 2) both have Hof of zero.

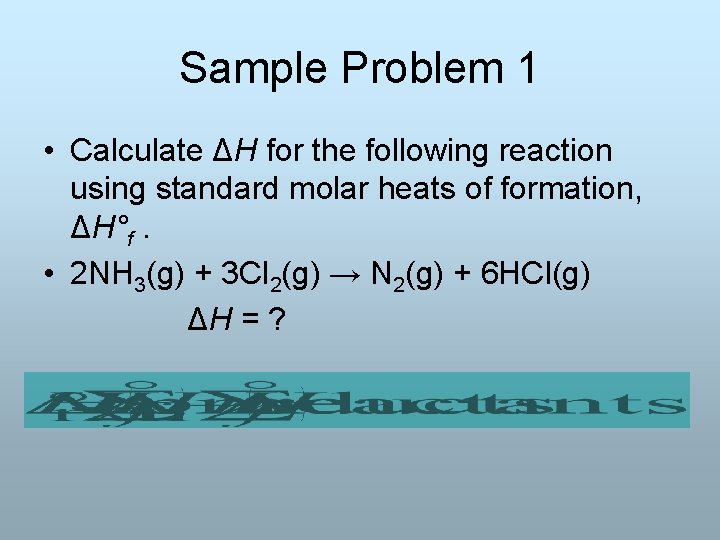
Sample Problem 1 • Calculate ΔH for the following reaction using standard molar heats of formation, ΔH°f. • 2 NH 3(g) + 3 Cl 2(g) → N 2(g) + 6 HCl(g) ΔH = ?

• ΔH°f for NH 3(g) = -45. 9 k. J/mol • ΔH°f for HCl(g) = -92. 3 k. J/mol • ΔH°f for Cl 2(g) and N 2(g) is 0 • ΔHrxn = Σ nΔH°f(product) - Σ nΔH°f(reactant) • ΔHrxn= (0 + 6(-92. 3 k. J)) - (2(-45. 9 k. J) + 0) = (-553 k. J) - (-91. 8 k. J) = -461 k. J

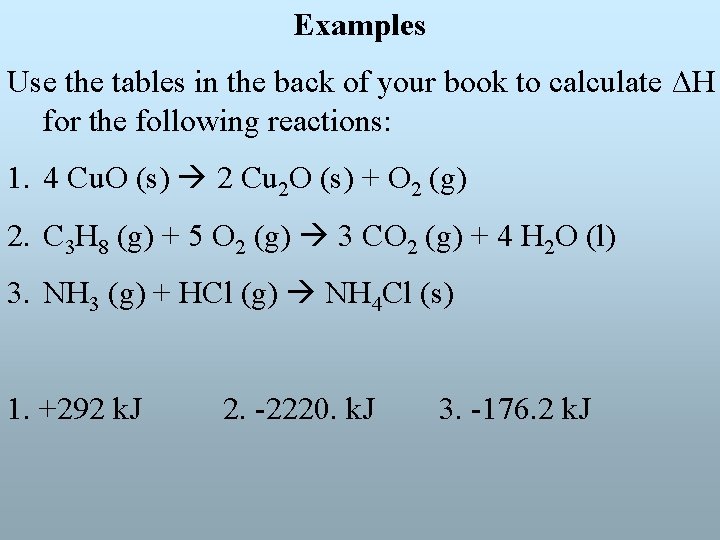
Examples Use the tables in the back of your book to calculate ΔH for the following reactions: 1. 4 Cu. O (s) 2 Cu 2 O (s) + O 2 (g) 2. C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (l) 3. NH 3 (g) + HCl (g) NH 4 Cl (s) 1. +292 k. J 2. -2220. k. J 3. -176. 2 k. J

Practice: • Page 685 #15 • Page 687 #19 -21 • Page 691 # 5
 17.4 calculating heats of reaction
17.4 calculating heats of reaction Calculating molar mass using colligative properties
Calculating molar mass using colligative properties Molarity to concentration
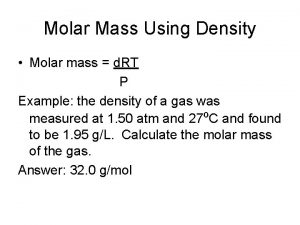
Molarity to concentration Molar mass from freezing point depression calculator
Molar mass from freezing point depression calculator Time of death estimations worksheet
Time of death estimations worksheet Limit of function
Limit of function Mechanism of death definition
Mechanism of death definition What is molar enthalpy
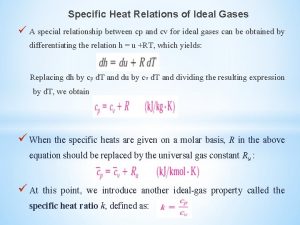
What is molar enthalpy Specific heat relations
Specific heat relations Internal energy of real gas
Internal energy of real gas Chapter 26 section 2 the cold war heats up answer key
Chapter 26 section 2 the cold war heats up answer key Chapter 18 section 2 guided reading the cold war heats up
Chapter 18 section 2 guided reading the cold war heats up