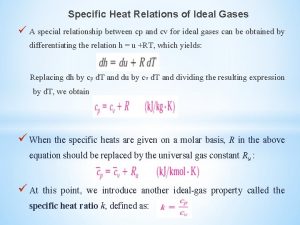
Heats of Combustion Heats of Formation Heats of

Heats of Combustion, Heats of Formation, Heats of Hydrogenation and Bond Dissociation Energies
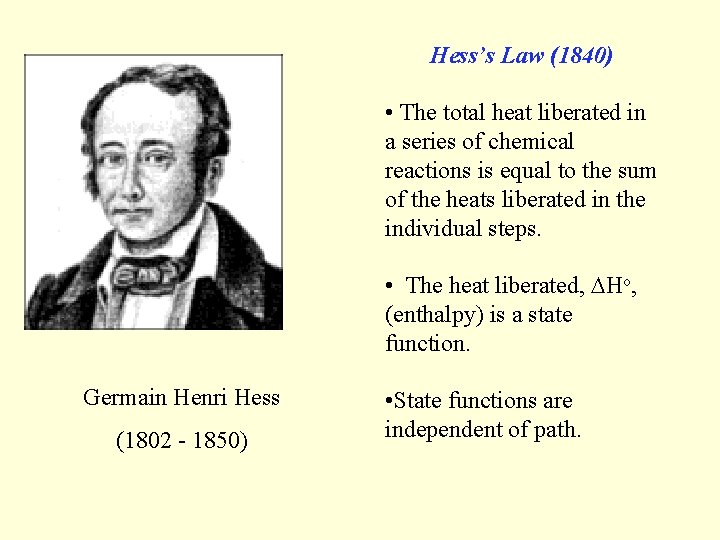
Hess’s Law (1840) • The total heat liberated in a series of chemical reactions is equal to the sum of the heats liberated in the individual steps. • The heat liberated, Ho, (enthalpy) is a state function. Germain Henri Hess (1802 - 1850) • State functions are independent of path.

The “Heat” Liberated in Either Route is the Same San Francisco Chicago Denver New Haven Dallas Miami
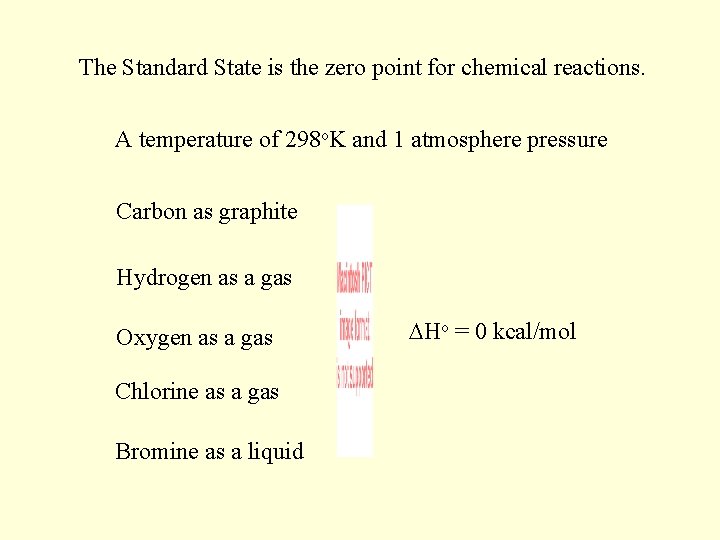
The Standard State is the zero point for chemical reactions. A temperature of 298 o. K and 1 atmosphere pressure Carbon as graphite Hydrogen as a gas Oxygen as a gas Chlorine as a gas Bromine as a liquid Ho = 0 kcal/mol
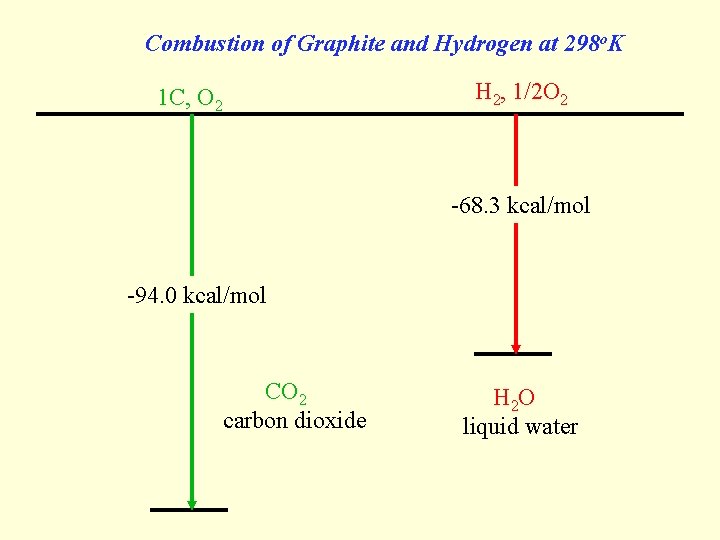
Combustion of Graphite and Hydrogen at 298 o. K H 2, 1/2 O 2 1 C, O 2 -68. 3 kcal/mol -94. 0 kcal/mol CO 2 carbon dioxide H 2 O liquid water
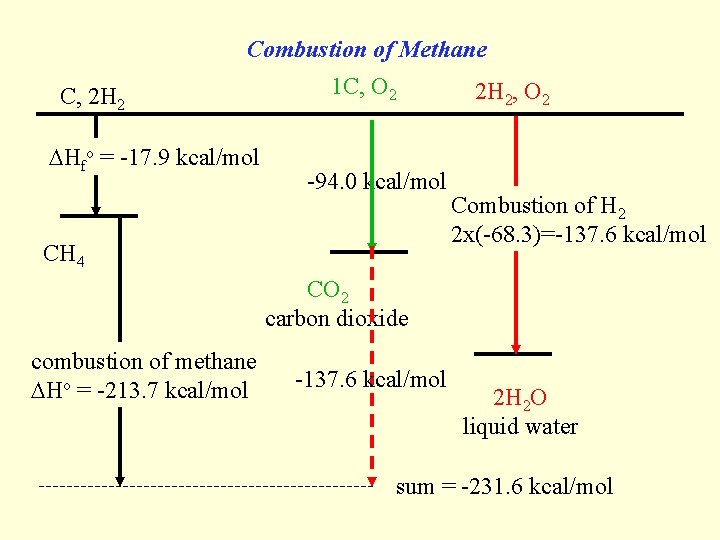
Combustion of Methane C, 2 H 2 Hfo = -17. 9 kcal/mol 1 C, O 2 -94. 0 kcal/mol CH 4 2 H 2, O 2 Combustion of H 2 2 x(-68. 3)=-137. 6 kcal/mol CO 2 carbon dioxide combustion of methane Ho = -213. 7 kcal/mol -137. 6 kcal/mol 2 H 2 O liquid water sum = -231. 6 kcal/mol
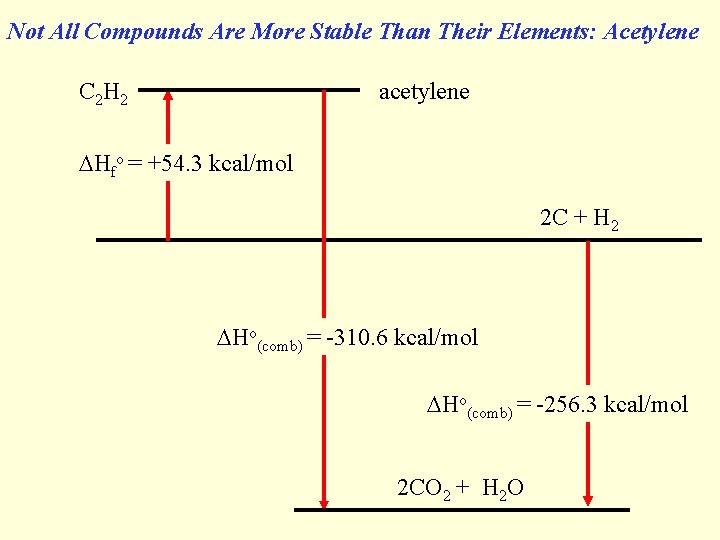
Not All Compounds Are More Stable Than Their Elements: Acetylene C 2 H 2 acetylene Hfo = +54. 3 kcal/mol 2 C + H 2 Ho(comb) = -310. 6 kcal/mol Ho(comb) = -256. 3 kcal/mol 2 CO 2 + H 2 O
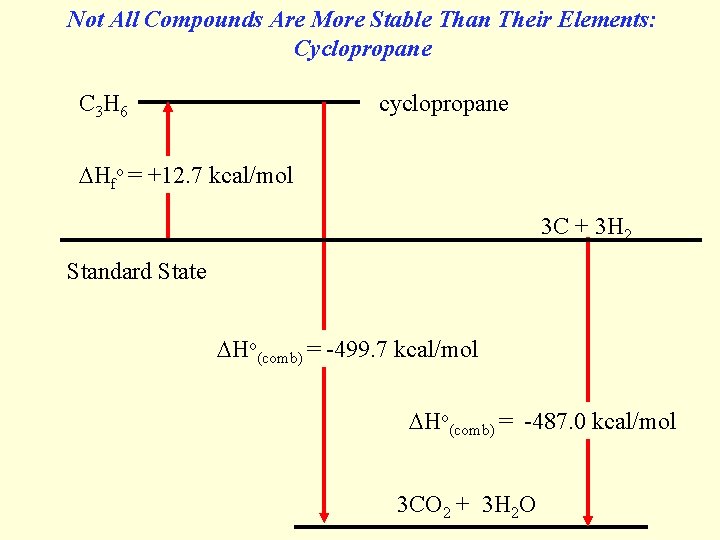
Not All Compounds Are More Stable Than Their Elements: Cyclopropane C 3 H 6 cyclopropane Hfo = +12. 7 kcal/mol 3 C + 3 H 2 Standard State Ho(comb) = -499. 7 kcal/mol Ho(comb) = -487. 0 kcal/mol 3 CO 2 + 3 H 2 O
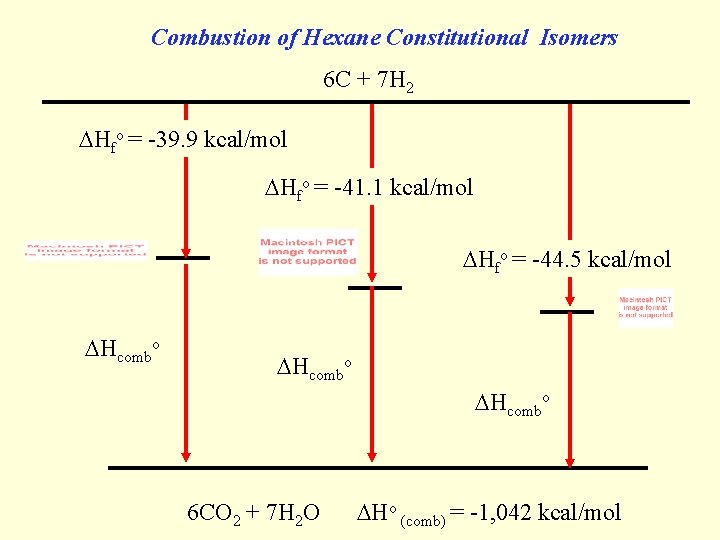
Combustion of Hexane Constitutional Isomers 6 C + 7 H 2 Hfo = -39. 9 kcal/mol Hfo = -41. 1 kcal/mol Hfo = -44. 5 kcal/mol Hcombo 6 CO 2 + 7 H 2 O Ho (comb) = -1, 042 kcal/mol
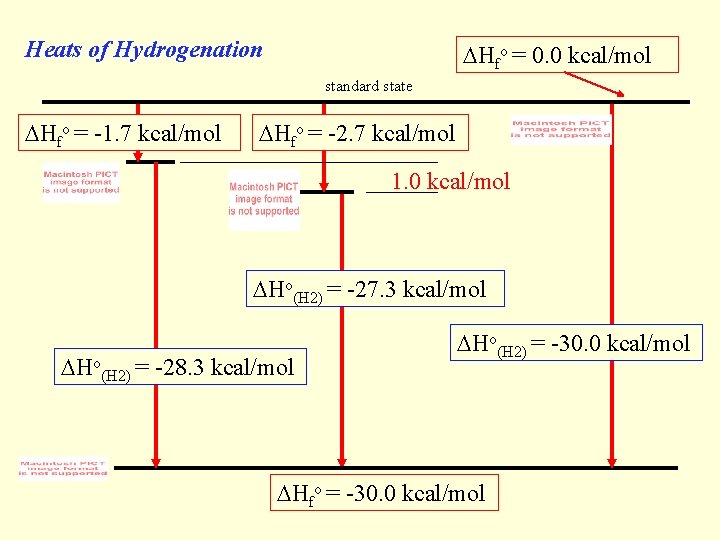
Heats of Hydrogenation Hfo = 0. 0 kcal/mol standard state Hfo = -1. 7 kcal/mol Hfo = -2. 7 kcal/mol 1. 0 kcal/mol Ho(H 2) = -27. 3 kcal/mol Ho(H 2) = -28. 3 kcal/mol Ho(H 2) = -30. 0 kcal/mol Hfo = -30. 0 kcal/mol
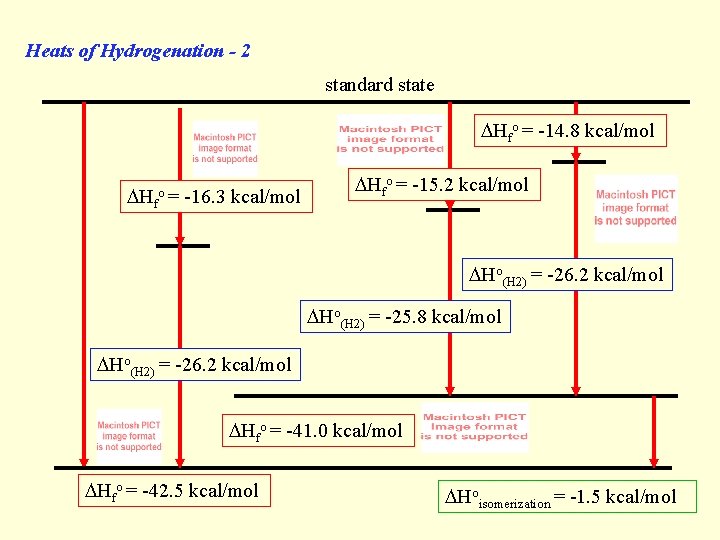
Heats of Hydrogenation - 2 standard state Hfo = -14. 8 kcal/mol Hfo = -16. 3 kcal/mol Hfo = -15. 2 kcal/mol Ho(H 2) = -26. 2 kcal/mol Ho(H 2) = -25. 8 kcal/mol Ho(H 2) = -26. 2 kcal/mol Hfo = -41. 0 kcal/mol Hfo = -42. 5 kcal/mol Hoisomerization = -1. 5 kcal/mol
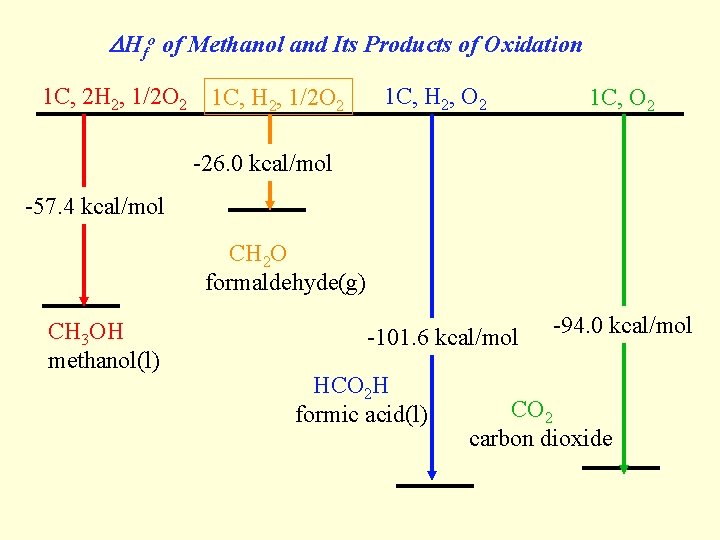
DHfo of Methanol and Its Products of Oxidation 1 C, 2 H 2, 1/2 O 2 1 C, H 2, O 2 1 C, O 2 -26. 0 kcal/mol -57. 4 kcal/mol CH 2 O formaldehyde(g) CH 3 OH methanol(l) -101. 6 kcal/mol HCO 2 H formic acid(l) -94. 0 kcal/mol CO 2 carbon dioxide
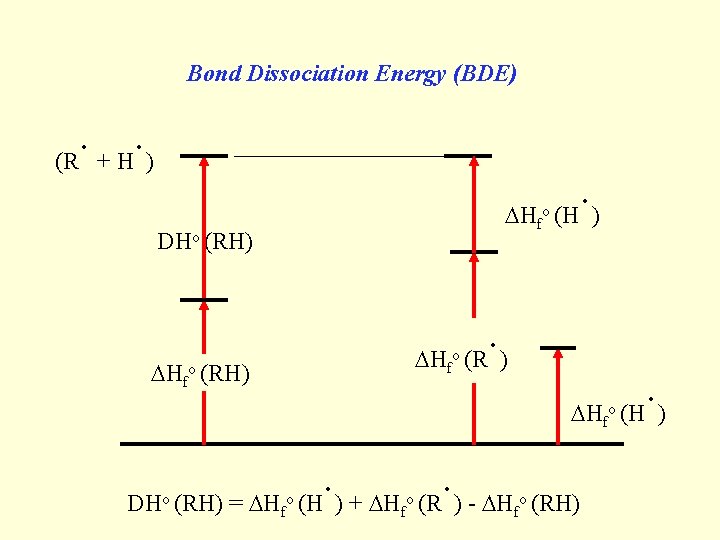
Bond Dissociation Energy (BDE) . . (R + H ) Hfo DHo (RH) Hfo o (RH) . (H ) . (R ) Hfo DHo (RH) = Hfo . . (H ) + H (R ) - H f o (RH) . (H )
Bond Dissociation Energy and Hybridization C-H HCCH DHo (kcal/mol) C-C(sp 3) CH 2 CH 3 C-sp 2 Carbon Hybridization C-sp 3 C-sp
BDEs of C-C, C-X and C-H Bonds DHo (kcal/mol) H Et Me Cl i-Pr t-Bu Br I Me Me Et Me i-Pr Alkyl Substituent t-Bu Me
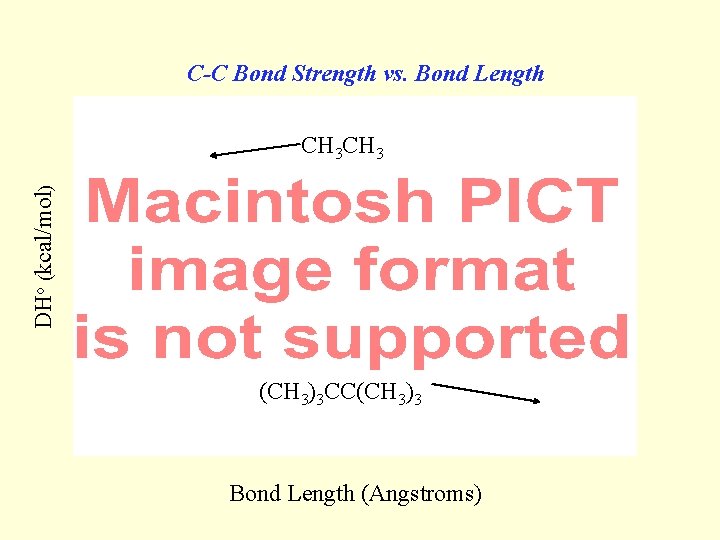
C-C Bond Strength vs. Bond Length DHo (kcal/mol) CH 3 (CH 3)3 CC(CH 3)3 Bond Length (Angstroms)
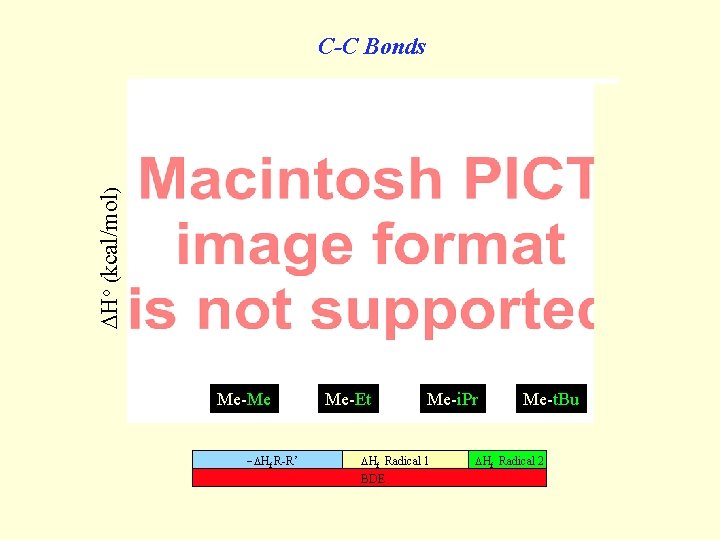
Ho (kcal/mol) C-C Bonds Me-Me Hf R-R’ Me-Et Me-i. Pr Hf Radical 1 BDE Me-t. Bu Hf Radical 2 t. Bu-t. Bu
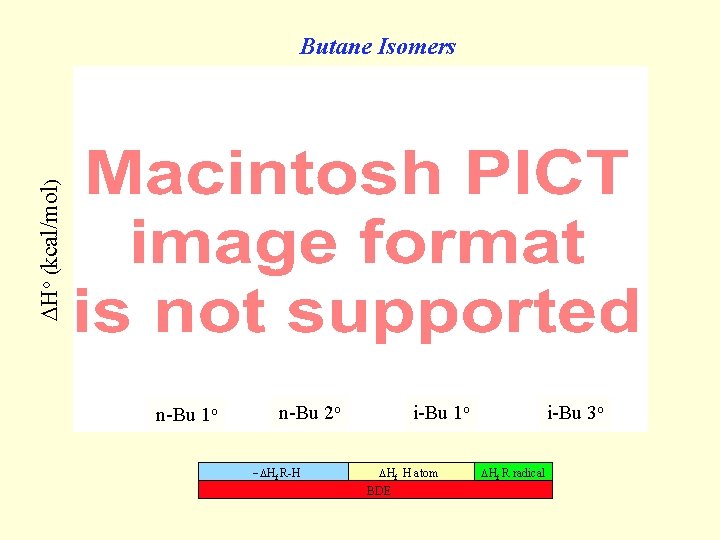
Ho (kcal/mol) Butane Isomers n-Bu 1 o n-Bu 2 o Hf R-H i-Bu 1 o Hf H atom BDE i-Bu 3 o Hf R radical

Ho (kcal/mol) Formation of C 4 -C 18 Even Primary Radicals from Their n-Alkanes Hf R-H Hf R radical BDE Hf H atom

The End
- Slides: 20