Fuels and Heats of Reactions Fuels and Heats

- Slides: 9

Fuels and Heats of Reactions

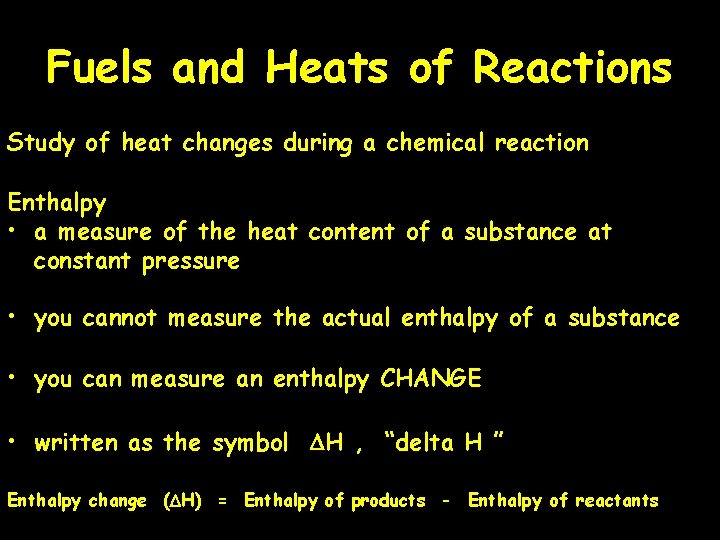
Fuels and Heats of Reactions Study of heat changes during a chemical reaction Enthalpy • a measure of the heat content of a substance at constant pressure • you cannot measure the actual enthalpy of a substance • you can measure an enthalpy CHANGE • written as the symbol DH , “delta H ” Enthalpy change (DH) = Enthalpy of products - Enthalpy of reactants

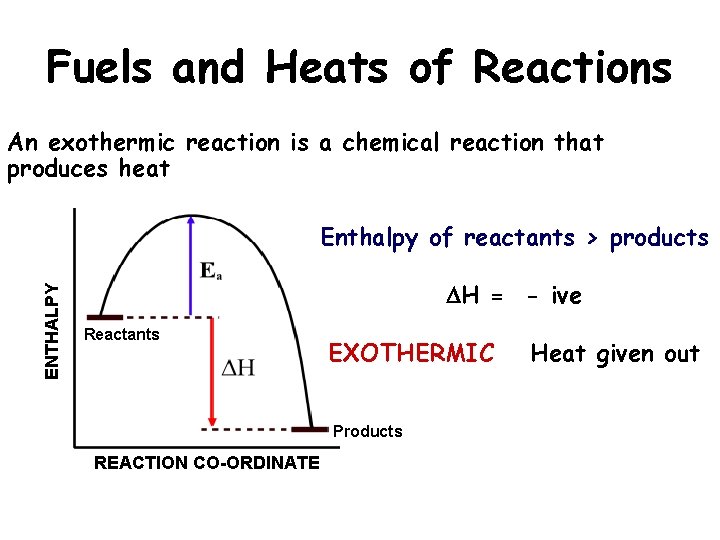
Fuels and Heats of Reactions An exothermic reaction is a chemical reaction that produces heat ENTHALPY Enthalpy of reactants > products DH = - ive Reactants EXOTHERMIC Products REACTION CO-ORDINATE Heat given out

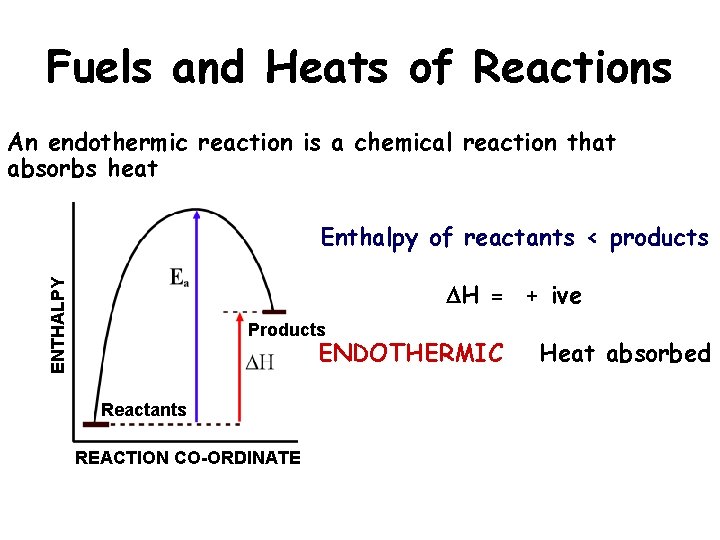
Fuels and Heats of Reactions An endothermic reaction is a chemical reaction that absorbs heat Enthalpy of reactants < products ENTHALPY DH = + ive Products ENDOTHERMIC Reactants REACTION CO-ORDINATE Heat absorbed

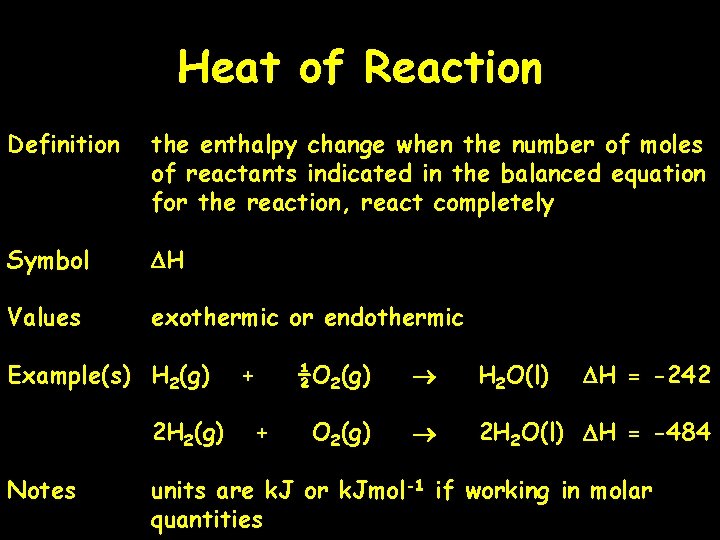
Heat of Reaction Definition the enthalpy change when the number of moles of reactants indicated in the balanced equation for the reaction, react completely Symbol DH Values exothermic or endothermic Example(s) H 2(g) 2 H 2(g) Notes + + ½O 2(g) H 2 O(l) DH = -242 O 2(g) 2 H 2 O(l) DH = -484 units are k. J or k. Jmol-1 if working in molar quantities

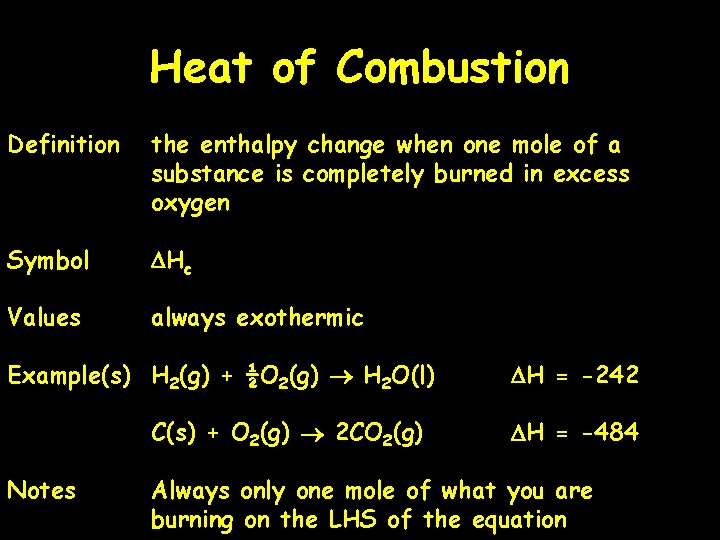
Heat of Combustion Definition the enthalpy change when one mole of a substance is completely burned in excess oxygen Symbol DHc Values always exothermic Example(s) H 2(g) + ½O 2(g) H 2 O(l) C(s) + O 2(g) 2 CO 2(g) Notes DH = -242 DH = -484 Always only one mole of what you are burning on the LHS of the equation

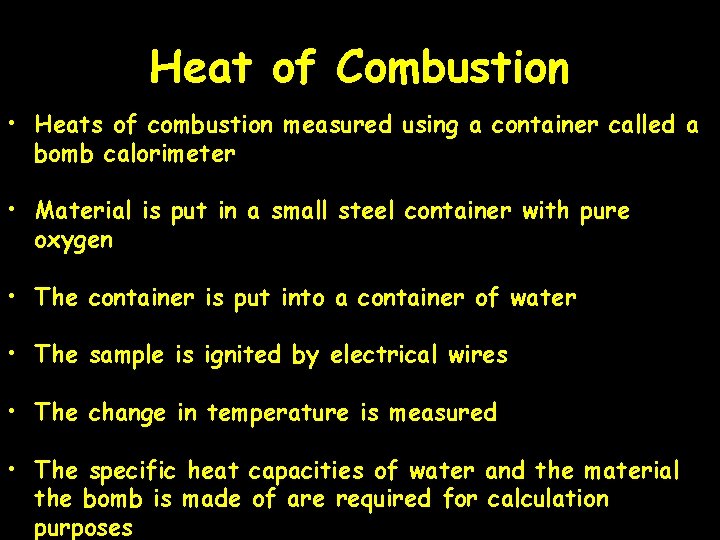
Heat of Combustion • Heats of combustion measured using a container called a bomb calorimeter • Material is put in a small steel container with pure oxygen • The container is put into a container of water • The sample is ignited by electrical wires • The change in temperature is measured • The specific heat capacities of water and the material the bomb is made of are required for calculation purposes

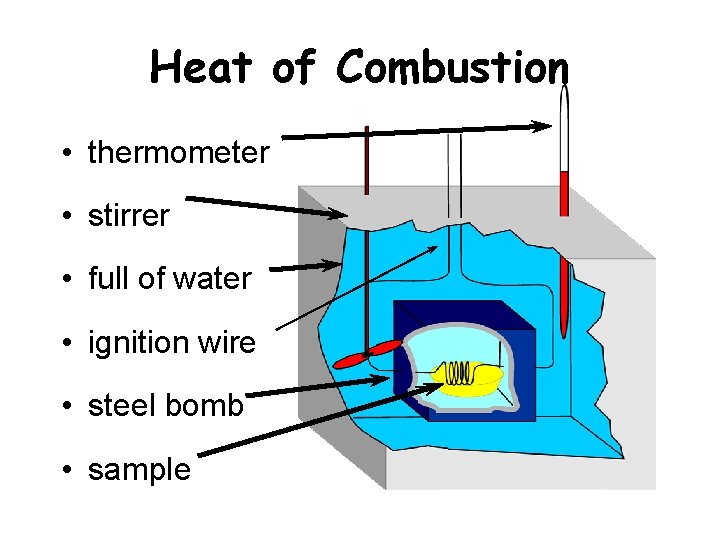
Heat of Combustion • thermometer • stirrer • full of water • ignition wire • steel bomb • sample

Heat of Combustion • Bomb calorimeters are also used to measure the efficiency of various fuels known as the Kilogram calorific value • The Kilogram calorific value of a fuel is the heat energy produced when 1 kg of the fuel is completely burned in oxygen
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Redox half reactions
Redox half reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Types of reactions
Types of reactions Unit 5 chemical reactions answers
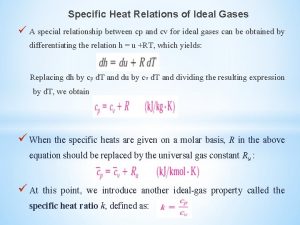
Unit 5 chemical reactions answers Specific heat relations
Specific heat relations Constant specific heats
Constant specific heats Chapter 26 section 2: the cold war heats up answer key
Chapter 26 section 2: the cold war heats up answer key Chapter 18 section 2 the cold war heats up
Chapter 18 section 2 the cold war heats up 17.4 calculating heats of reaction
17.4 calculating heats of reaction