17 4 Calculating Heats of Reaction Chapter 17






- Slides: 39

17. 4 Calculating Heats of Reaction > Chapter 17 Thermochemistry 17. 1 The Flow of Energy 17. 2 Measuring and Expressing Enthalpy Changes 17. 3 Heat in Changes of State 17. 4 Calculating Heats of Reaction 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

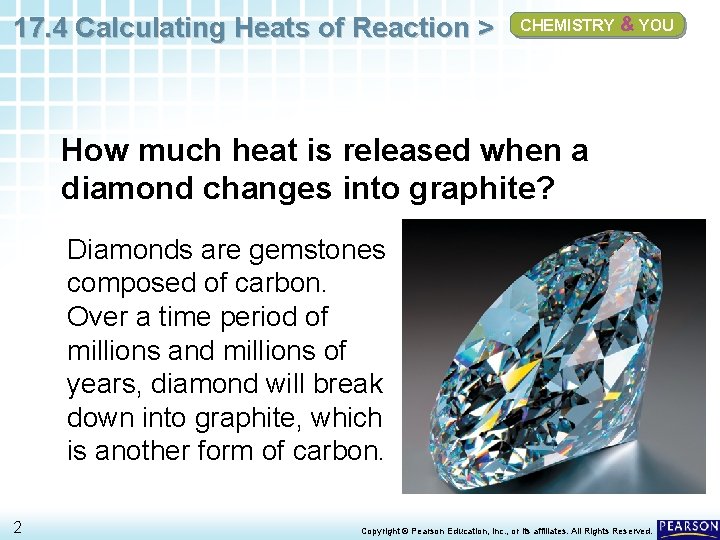
17. 4 Calculating Heats of Reaction > CHEMISTRY & YOU How much heat is released when a diamond changes into graphite? Diamonds are gemstones composed of carbon. Over a time period of millions and millions of years, diamond will break down into graphite, which is another form of carbon. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Hess’s Law How can you calculate the heat of reaction when it cannot be directly measured? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

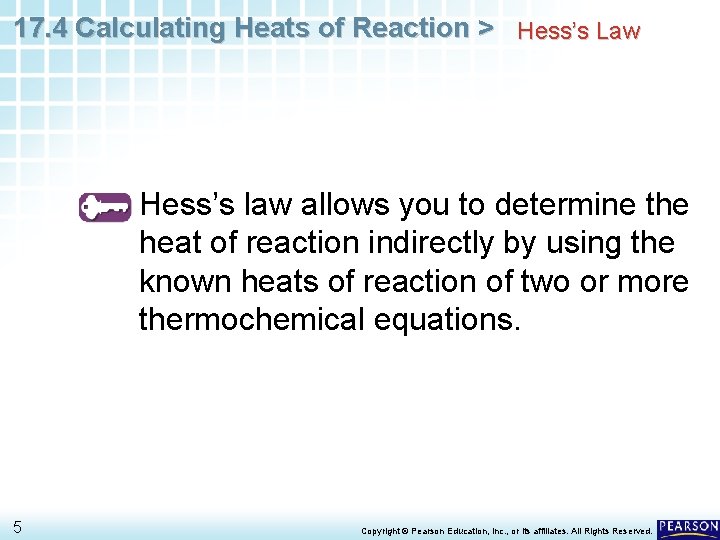
17. 4 Calculating Heats of Reaction > Hess’s Law Hess’s law of heat summation states that if you add two or more thermochemical equations to give a final equation, then you can also add the heats of reaction to give the final heat of reaction. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

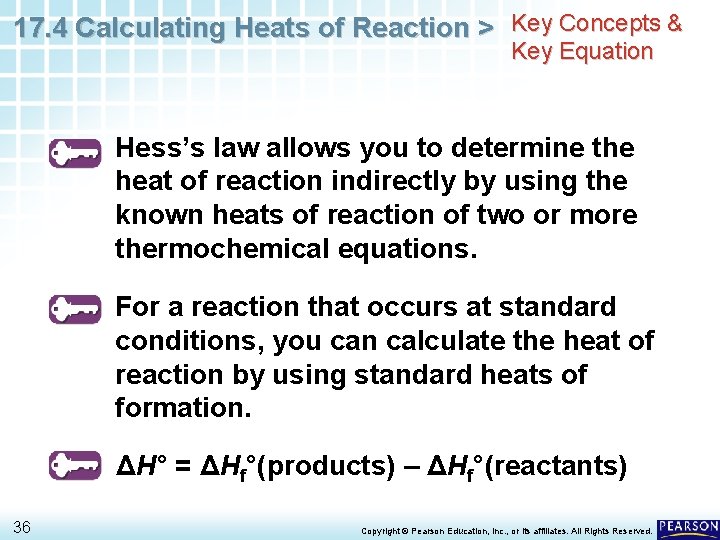
17. 4 Calculating Heats of Reaction > Hess’s Law Hess’s law allows you to determine the heat of reaction indirectly by using the known heats of reaction of two or more thermochemical equations. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

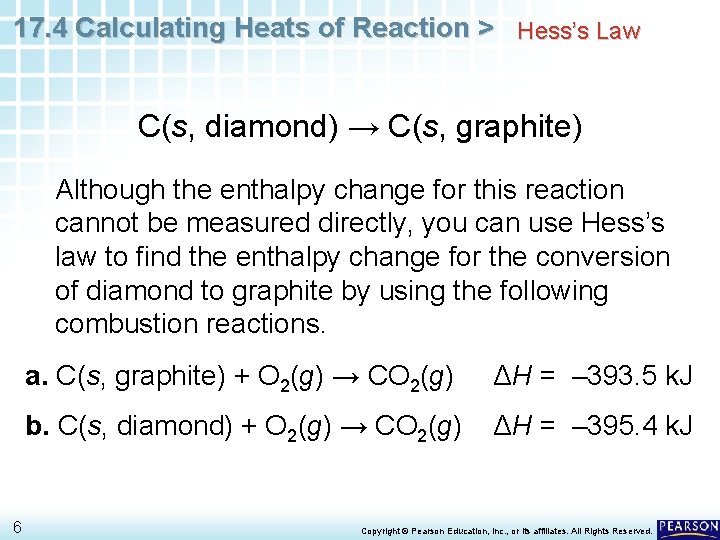
17. 4 Calculating Heats of Reaction > Hess’s Law C(s, diamond) → C(s, graphite) Although the enthalpy change for this reaction cannot be measured directly, you can use Hess’s law to find the enthalpy change for the conversion of diamond to graphite by using the following combustion reactions. 6 a. C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J b. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

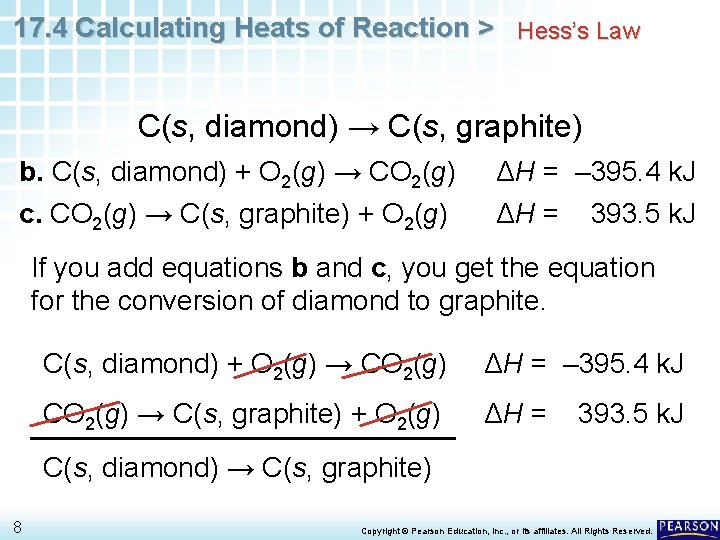
17. 4 Calculating Heats of Reaction > Hess’s Law C(s, diamond) → C(s, graphite) a. C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J b. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J Write equation a in reverse to give: c. CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J When you reverse a reaction, you must also change the sign of ΔH. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

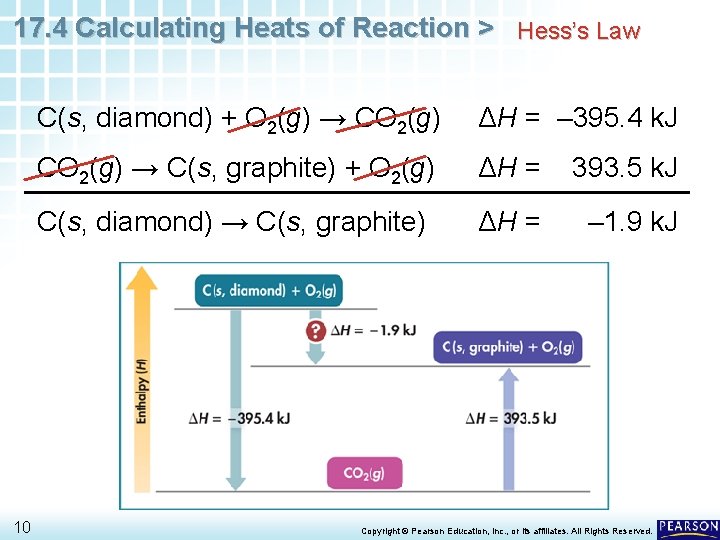
17. 4 Calculating Heats of Reaction > Hess’s Law C(s, diamond) → C(s, graphite) b. C(s, diamond) + O 2(g) → CO 2(g) c. CO 2(g) → C(s, graphite) + O 2(g) ΔH = – 395. 4 k. J ΔH = 393. 5 k. J If you add equations b and c, you get the equation for the conversion of diamond to graphite. C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

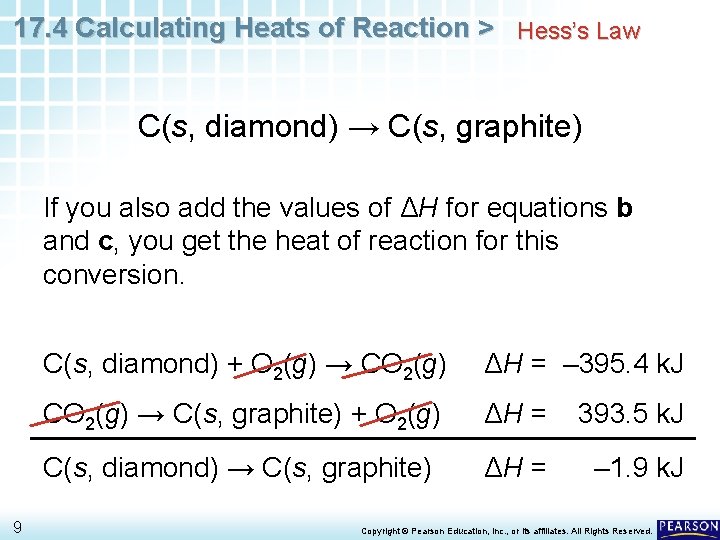
17. 4 Calculating Heats of Reaction > Hess’s Law C(s, diamond) → C(s, graphite) If you also add the values of ΔH for equations b and c, you get the heat of reaction for this conversion. 9 C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) ΔH = – 1. 9 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Hess’s Law 10 C(s, diamond) + O 2(g) → CO 2(g) ΔH = – 395. 4 k. J CO 2(g) → C(s, graphite) + O 2(g) ΔH = 393. 5 k. J C(s, diamond) → C(s, graphite) ΔH = – 1. 9 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

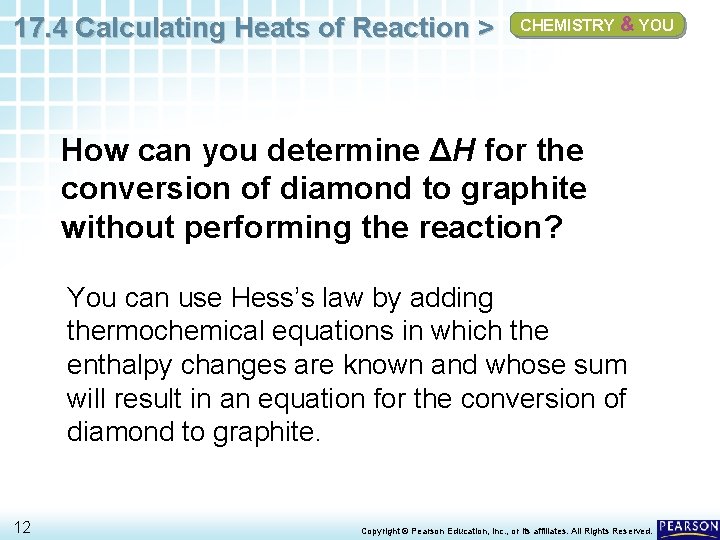
17. 4 Calculating Heats of Reaction > CHEMISTRY & YOU How can you determine ΔH for the conversion of diamond to graphite without performing the reaction? 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > CHEMISTRY & YOU How can you determine ΔH for the conversion of diamond to graphite without performing the reaction? You can use Hess’s law by adding thermochemical equations in which the enthalpy changes are known and whose sum will result in an equation for the conversion of diamond to graphite. 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

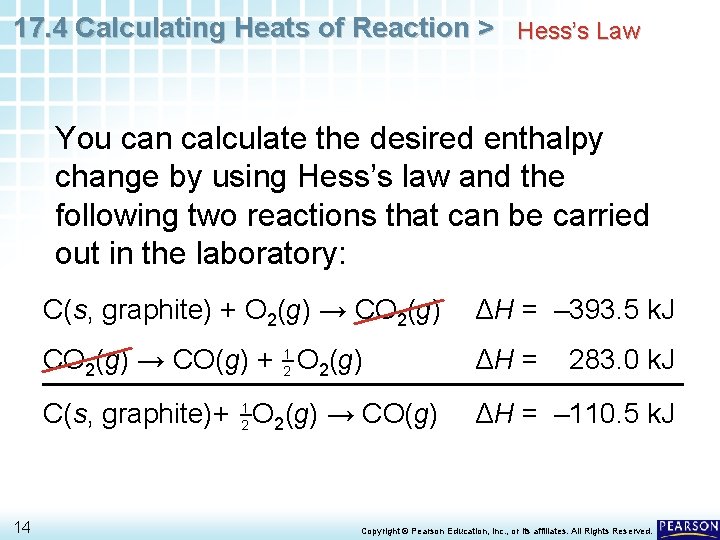
17. 4 Calculating Heats of Reaction > Hess’s Law Another case where Hess’s law is useful is when reactions yield products in addition to the product of interest. • Suppose you want to determine the enthalpy change for the formation of carbon monoxide from its elements. C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = ? • Carrying out the reaction in the laboratory as written is virtually impossible. 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

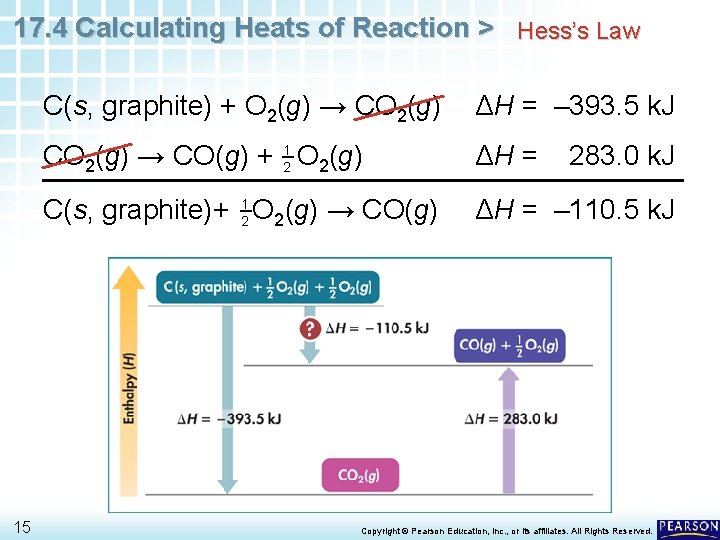
17. 4 Calculating Heats of Reaction > Hess’s Law You can calculate the desired enthalpy change by using Hess’s law and the following two reactions that can be carried out in the laboratory: 14 C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J CO 2(g) → CO(g) + 21 O 2(g) ΔH = C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = – 110. 5 k. J 283. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Hess’s Law 15 C(s, graphite) + O 2(g) → CO 2(g) ΔH = – 393. 5 k. J CO 2(g) → CO(g) + 21 O 2(g) ΔH = C(s, graphite)+ 21 O 2(g) → CO(g) ΔH = – 110. 5 k. J 283. 0 k. J Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > According to Hess’s law, it is possible to calculate an unknown heat of reaction by using which of the following? A. heats of fusion for each of the compounds in the reaction B. two other reactions with known heats of reaction C. specific heat capacities for each compound in the reaction D. density for each compound in the reaction 16 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > According to Hess’s law, it is possible to calculate an unknown heat of reaction by using which of the following? A. heats of fusion for each of the compounds in the reaction B. two other reactions with known heats of reaction C. specific heat capacities for each compound in the reaction D. density for each compound in the reaction 17 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Standard Heats of Formation How can you calculate the heat of reaction when it cannot be directly measured? 18 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Standard Heats of Formation Enthalpy changes generally depend on the conditions of the process. • Scientists specify a common set of conditions as a reference point. • These conditions, called the standard state, refer to the stable form of a substance at 25°C and 101. 3 k. Pa. 19 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

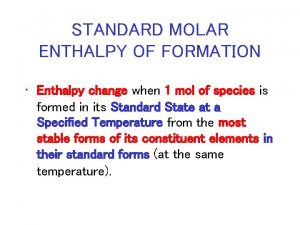
17. 4 Calculating Heats of Reaction > Standard Heats of Formation The standard heat of formation (ΔHf°) of a compound is the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states. • The ΔHf° of a free element in its standard state is arbitrarily set at zero. • Thus, ΔHf° = 0 for the diatomic molecules H 2(g), N 2(g), O 2(g), F 2(g), Cl 2(g), Br 2(l), and I 2(s). 20 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

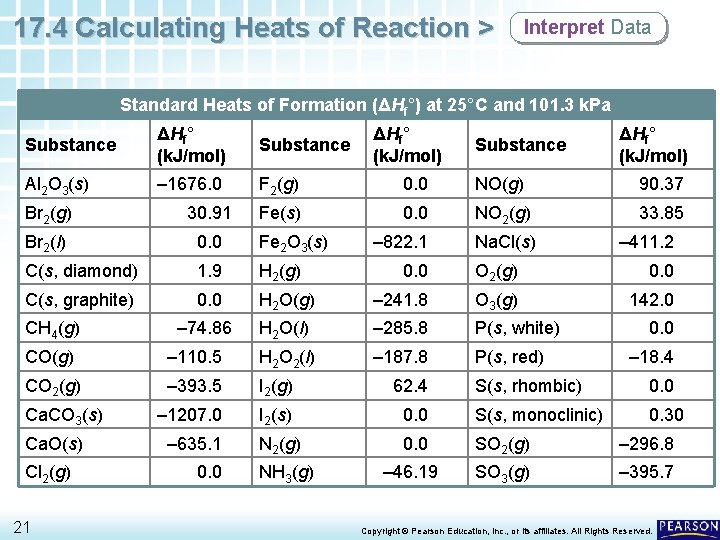
17. 4 Calculating Heats of Reaction > Interpret Data Standard Heats of Formation (ΔHf°) at 25°C and 101. 3 k. Pa Substance ΔHf° (k. J/mol) Substance Al 2 O 3(s) – 1676. 0 F 2(g) 0. 0 NO(g) 90. 37 Fe(s) 0. 0 NO 2(g) 33. 85 – 822. 1 Na. Cl(s) Br 2(g) 30. 91 Br 2(l) 0. 0 Fe 2 O 3(s) C(s, diamond) 1. 9 H 2(g) C(s, graphite) 0. 0 – 74. 86 CH 4(g) ΔHf° (k. J/mol) 0. 0 H 2 O(g) – 241. 8 O 3(g) 142. 0 H 2 O(l) – 285. 8 P(s, white) – 187. 8 P(s, red) H 2 O 2(l) CO 2(g) – 393. 5 I 2(g) 62. 4 – 1207. 0 I 2(s) – 635. 1 N 2(g) Cl 2(g) 21 0. 0 – 411. 2 O 2(g) – 110. 5 Ca. O(s) ΔHf° (k. J/mol) 0. 0 CO(g) Ca. CO 3(s) Substance NH 3(g) 0. 0 – 18. 4 S(s, rhombic) 0. 0 S(s, monoclinic) 0. 30 0. 0 SO 2(g) – 296. 8 – 46. 19 SO 3(g) – 395. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. 22 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

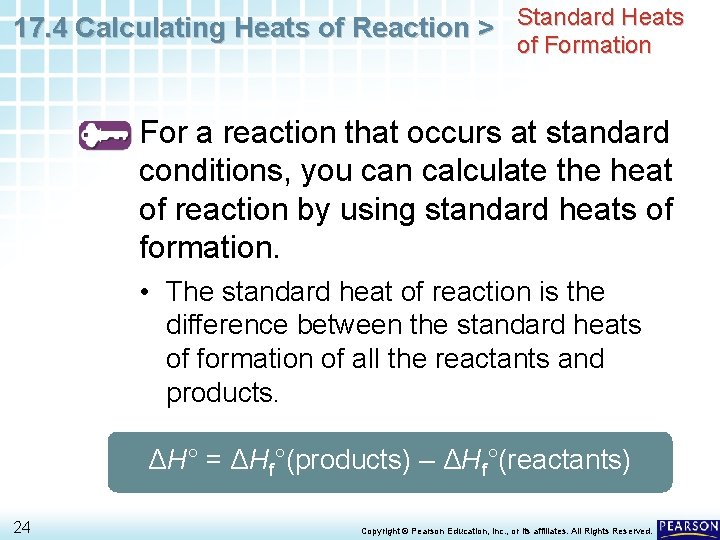
17. 4 Calculating Heats of Reaction > Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. • Such an enthalpy change is called the standard heat of reaction (ΔH°). 23 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Standard Heats of Formation For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. • The standard heat of reaction is the difference between the standard heats of formation of all the reactants and products. ΔH° = ΔHf°(products) – ΔHf°(reactants) 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

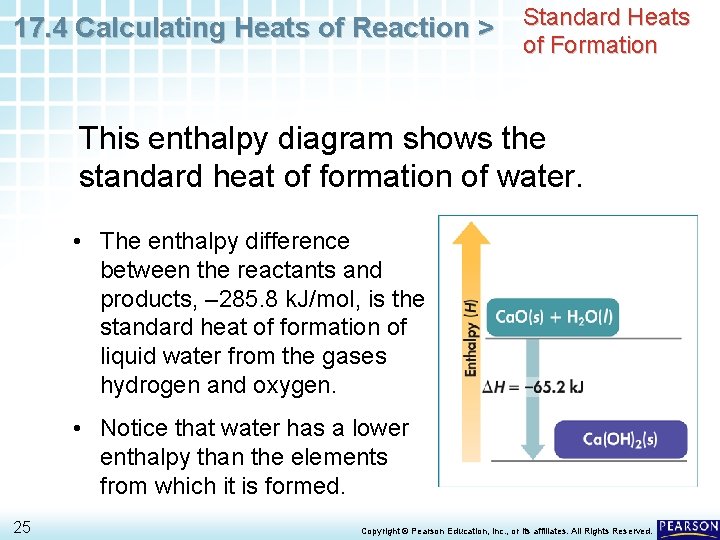
17. 4 Calculating Heats of Reaction > Standard Heats of Formation This enthalpy diagram shows the standard heat of formation of water. • The enthalpy difference between the reactants and products, – 285. 8 k. J/mol, is the standard heat of formation of liquid water from the gases hydrogen and oxygen. • Notice that water has a lower enthalpy than the elements from which it is formed. 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 Calculating the Standard Heat of Reaction What is the standard heat of reaction (ΔH°) for the reaction of CO(g) with O 2(g) to form CO 2(g)? 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

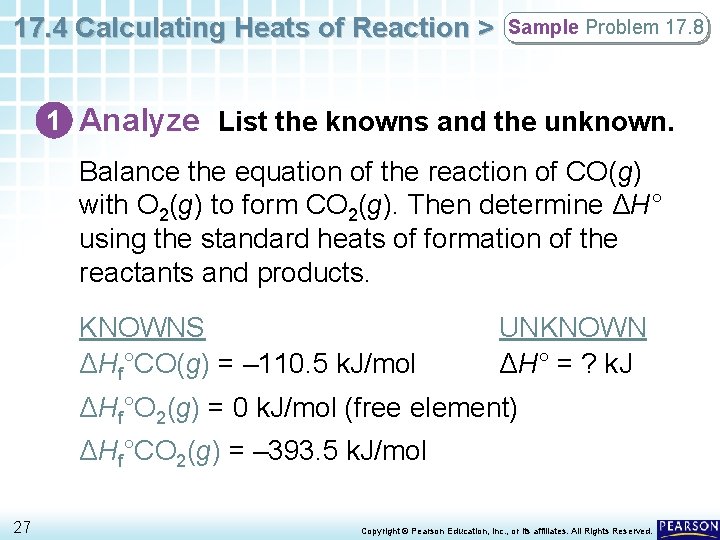
17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 1 Analyze List the knowns and the unknown. Balance the equation of the reaction of CO(g) with O 2(g) to form CO 2(g). Then determine ΔH° using the standard heats of formation of the reactants and products. KNOWNS ΔHf°CO(g) = – 110. 5 k. J/mol UNKNOWN ΔH° = ? k. J ΔHf°O 2(g) = 0 k. J/mol (free element) ΔHf°CO 2(g) = – 393. 5 k. J/mol 27 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

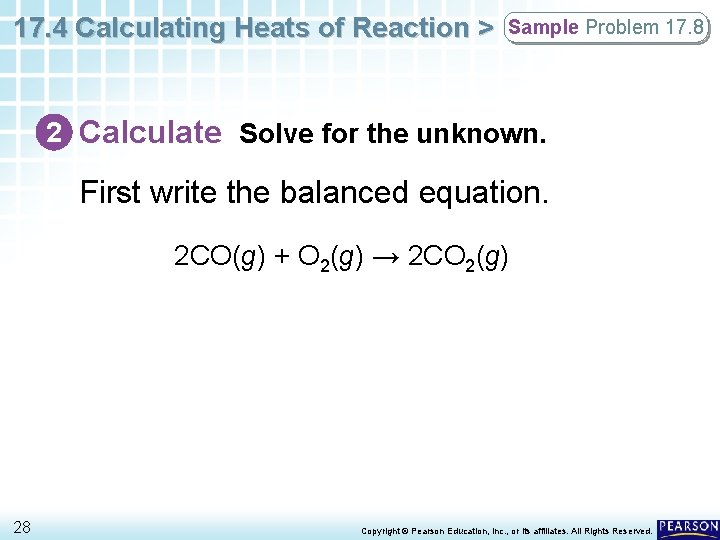
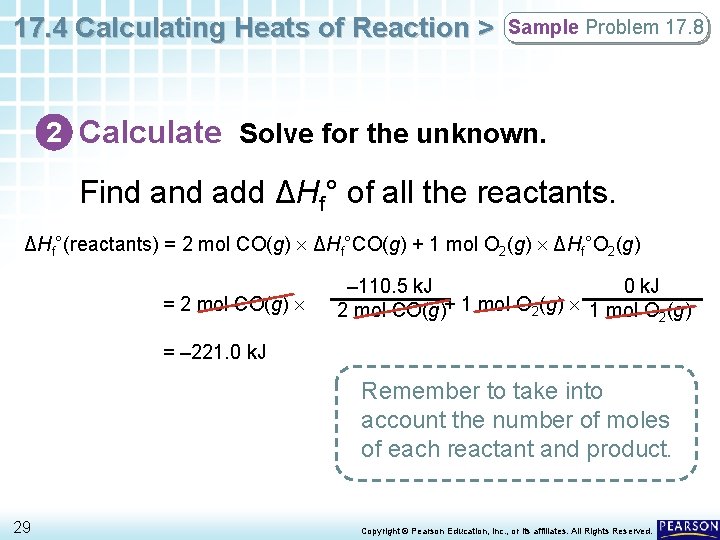
17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 2 Calculate Solve for the unknown. First write the balanced equation. 2 CO(g) + O 2(g) → 2 CO 2(g) 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 2 Calculate Solve for the unknown. Find add ΔHf° of all the reactants. ΔHf°(reactants) = 2 mol CO(g) ΔHf°CO(g) + 1 mol O 2(g) ΔHf°O 2(g) = 2 mol CO(g) – 110. 5 k. J 0 k. J 2 mol CO(g)+ 1 mol O 2(g) = – 221. 0 k. J Remember to take into account the number of moles of each reactant and product. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

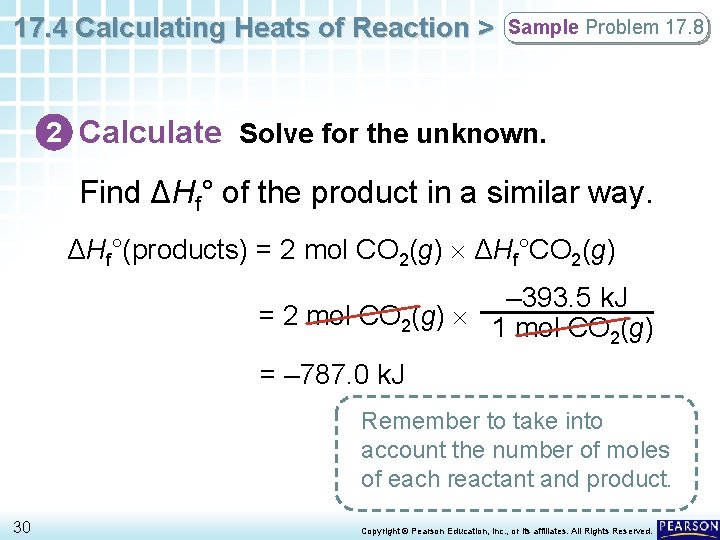
17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 2 Calculate Solve for the unknown. Find ΔHf° of the product in a similar way. ΔHf°(products) = 2 mol CO 2(g) ΔHf°CO 2(g) – 393. 5 k. J = 2 mol CO 2(g) 1 mol CO (g) 2 = – 787. 0 k. J Remember to take into account the number of moles of each reactant and product. 30 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

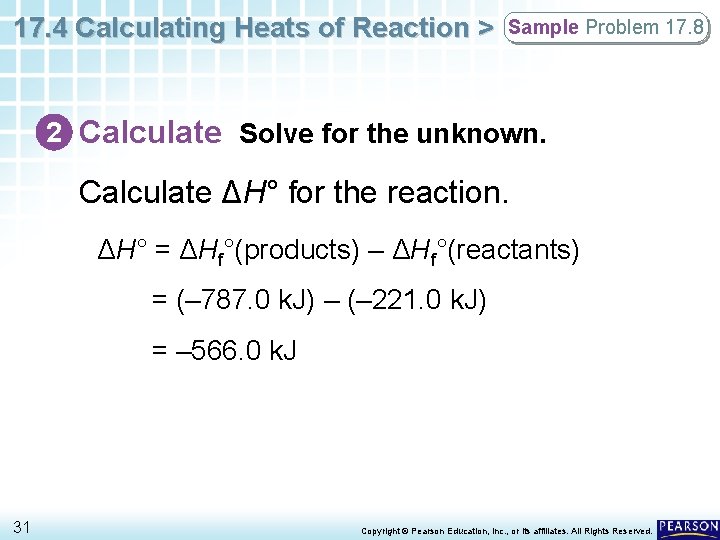
17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 2 Calculate Solve for the unknown. Calculate ΔH° for the reaction. ΔH° = ΔHf°(products) – ΔHf°(reactants) = (– 787. 0 k. J) – (– 221. 0 k. J) = – 566. 0 k. J 31 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

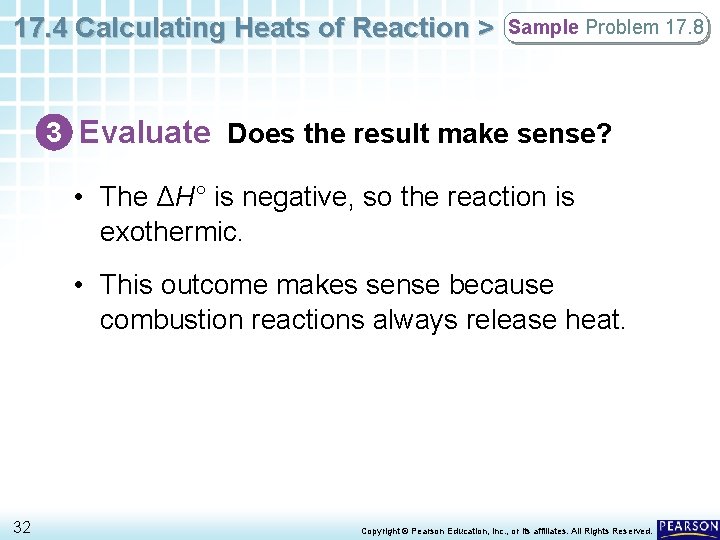
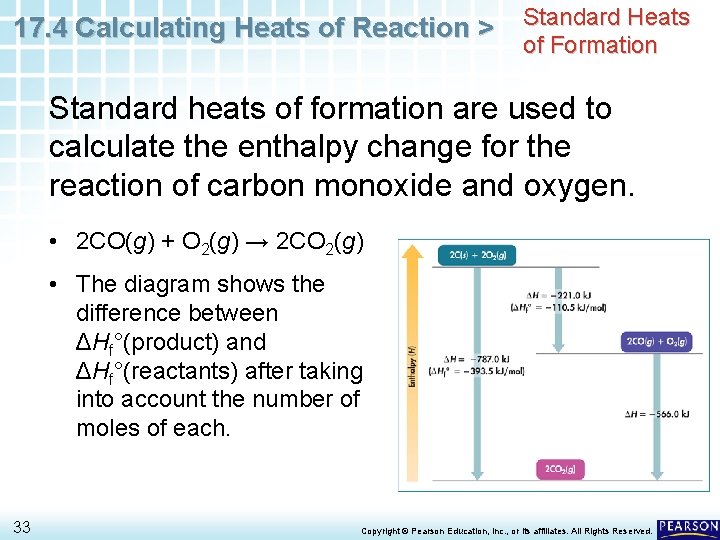
17. 4 Calculating Heats of Reaction > Sample Problem 17. 8 3 Evaluate Does the result make sense? • The ΔH° is negative, so the reaction is exothermic. • This outcome makes sense because combustion reactions always release heat. 32 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Standard Heats of Formation Standard heats of formation are used to calculate the enthalpy change for the reaction of carbon monoxide and oxygen. • 2 CO(g) + O 2(g) → 2 CO 2(g) • The diagram shows the difference between ΔHf°(product) and ΔHf°(reactants) after taking into account the number of moles of each. 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

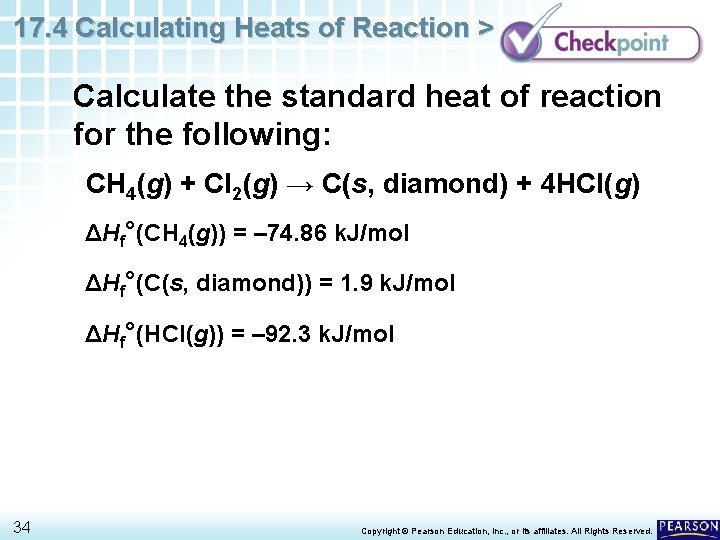
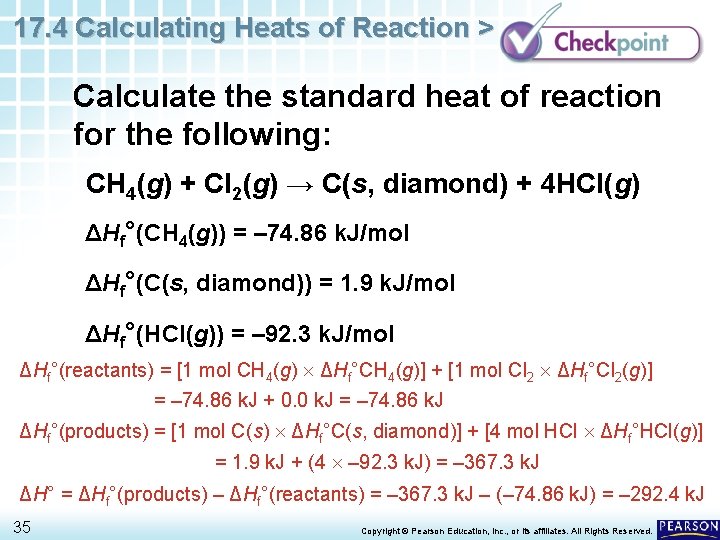
17. 4 Calculating Heats of Reaction > Calculate the standard heat of reaction for the following: CH 4(g) + Cl 2(g) → C(s, diamond) + 4 HCl(g) ΔHf°(CH 4(g)) = – 74. 86 k. J/mol ΔHf°(C(s, diamond)) = 1. 9 k. J/mol ΔHf°(HCl(g)) = – 92. 3 k. J/mol 34 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Calculate the standard heat of reaction for the following: CH 4(g) + Cl 2(g) → C(s, diamond) + 4 HCl(g) ΔHf°(CH 4(g)) = – 74. 86 k. J/mol ΔHf°(C(s, diamond)) = 1. 9 k. J/mol ΔHf°(HCl(g)) = – 92. 3 k. J/mol ΔHf°(reactants) = [1 mol CH 4(g) ΔHf°CH 4(g)] + [1 mol Cl 2 ΔHf°Cl 2(g)] = – 74. 86 k. J + 0. 0 k. J = – 74. 86 k. J ΔHf°(products) = [1 mol C(s) ΔHf°C(s, diamond)] + [4 mol HCl ΔHf°HCl(g)] = 1. 9 k. J + (4 – 92. 3 k. J) = – 367. 3 k. J ΔH° = ΔHf°(products) – ΔHf°(reactants) = – 367. 3 k. J – (– 74. 86 k. J) = – 292. 4 k. J 35 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Key Concepts & Key Equation Hess’s law allows you to determine the heat of reaction indirectly by using the known heats of reaction of two or more thermochemical equations. For a reaction that occurs at standard conditions, you can calculate the heat of reaction by using standard heats of formation. ΔH° = ΔHf°(products) – ΔHf°(reactants) 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > Glossary Terms • Hess’s law of heat summation: if you add two or more thermochemical equations to give a final equation, then you also add the heats of reaction to give the final heat of reaction • standard heat of formation (ΔHf°): the change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states at 25°C 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > BIG IDEA Matter and Energy The heat of reaction can be calculated by using the known heats of reaction of two or more thermochemical equations or by using standard heats of formation. 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

17. 4 Calculating Heats of Reaction > END OF 17. 4 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.
 17.4 calculating heats of reaction
17.4 calculating heats of reaction Chapter 26 section 2: the cold war heats up answer key
Chapter 26 section 2: the cold war heats up answer key Chapter 18 section 2 the cold war heats up
Chapter 18 section 2 the cold war heats up Enthalpy change of solution
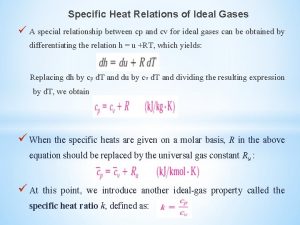
Enthalpy change of solution Cv and cp relationship
Cv and cp relationship Internal energy of real gas
Internal energy of real gas Truman speech
Truman speech The cold war heats up: 1945 - 1969
The cold war heats up: 1945 - 1969 Reaction rate formula
Reaction rate formula E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Activity formula
Activity formula Calculate percentage yield
Calculate percentage yield How to calculate theoretical yield
How to calculate theoretical yield Calculate unemployment rate
Calculate unemployment rate How to calculate the percent difference
How to calculate the percent difference Average 400 meter time
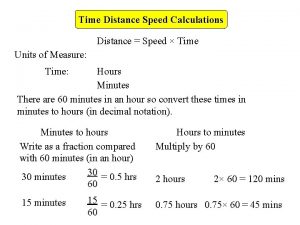
Average 400 meter time How to find time with distance and speed
How to find time with distance and speed Calculating gdp formula
Calculating gdp formula A cart travels with a constant nonzero acceleration
A cart travels with a constant nonzero acceleration Standard error of difference
Standard error of difference Light microscopes uses
Light microscopes uses Abi test
Abi test Speed distance formula
Speed distance formula Hubbart formula steps
Hubbart formula steps Calculating nnt
Calculating nnt Molar heat of formation
Molar heat of formation Total distance formula
Total distance formula How to get speed from distance and time
How to get speed from distance and time Daily compound interest formula
Daily compound interest formula Dilution factor
Dilution factor Calculating ka and kb
Calculating ka and kb Calculating oxidation state
Calculating oxidation state Pack year
Pack year Net operating profit after tax
Net operating profit after tax Percentage decrease calculator
Percentage decrease calculator Mass by mass percentage formula
Mass by mass percentage formula Calculating half life
Calculating half life Calculating food cost
Calculating food cost Calculate the molarity
Calculate the molarity