Atoms Lynn A Melton University of Texas at



























- Slides: 34

Atoms! Lynn A. Melton University of Texas at Dallas Mini-CAST February 21, 2009 02 -21 -09 1

Website • http: //www. chemchapterzero. com • Lots of ideas there. • It you use this material in the classroom, it may take you a month or more to work through the material. . • The “hands on” stuff will count as labs. • No algebra! 02 -21 -09 2

Fundamental Concepts of Chemistry • Atoms • Bonding/Molecules/Reactions • Structure/Properties – Activity of molecule derives from its structure 02 -21 -09 3

Some Fundamental Skills • Ability to work with models • “Seeing without seeing” 02 -21 -09 4

#1 Models A model is a step on the staircase of understanding 02 -21 -09 5

#1 Models Working scientists use many of the steps. They use the simplest model that works, since the higher steps generally require more complex mathematics. 02 -21 -09 6

#1 Models are generally not completely “true”. They generally explains some things well and other things poorly. 02 -21 -09 7

#1 Models Which step is best for us? 02 -21 -09 8

#2 Seeing Without Seeing • Most chemists see atoms moving when they talk about reactions. Maybe they have a “tv screen” in the front of their brain. • It takes students a long time (sophomore year of college? ) to acquire this skill. • How can we intentionally start to build this skill in younger students? 02 -21 -09 9

#3 Atoms • A conceptual chemistry problem: • “A piece of normal (dirty) copper wire is held in a gas flame. It becomes bright copper “pink”. When it is removed from the flame and allowed to cool, it becomes black. • Question: Does the blackened copper wire weigh more, same, or less than when it was in the flame? ” 02 -21 -09 10

#3 Atoms • A secondary school teacher was in the class, and came to me for help with this homework problem. • She could tell me that the flame cleaned the surface of the dirty copper wire and that oxygen from the air reacted with the clean surface to produce copper oxide, which is black. • She went back and forth as to whether the answer was “more”, “same”, or “less”. She was guessing. 02 -21 -09 11

#3 Atoms • I tried to help. Knowing that she once had taught Home Economics, I said, “Go to the grocery store and fill a basket with oranges. Now put a layer of avocados on top of the oranges. Does the basket weigh more, same or less when I add the avocados? • “Oh, Dr. Melton, of course it weighs more”. • She could reasons well enough, but when she was asked about atoms, she turned off her reasoning. • The atomic world was ARCANE. 02 -21 -09 12

#3 Atoms • The atomic world was ARCANE. – Known or understood by only a few: arcane economic theories. – adj : requiring secret or mysterious knowledge; "the arcane science of dowsing“ – Definitions from dictionary. com • In the arcane world, the normal rules do not work, and you might as well guess. 02 -21 -09 13

#3 Atoms • If a sassy ninth grader asked you “So why – other than you and the book say so – should I accept that the world is made of atoms? After all, I cannot see atoms. ” 02 -21 -09 14

#3 Atoms • Your answer has three parts: – Define an atom carefully – Data #1: Atomic Force Microscopy (in #5) (the world is granular) – Data #2: Mass Spectrometry (in #5) (the particles have different weights) 02 -21 -09 15

#3 Atoms • Definition of an atom – Rip any piece of the world apart, but you may use only the energies available to the ancients – horses, flames, and lightning. When you cannot rip the smaller pieces apart any longer (to produce only neutral particles) then those last (neutral) particles are ATOMS. 02 -21 -09 16

#3 Atoms • The weight of anything in the world is the same, regardless of how finely you divide it. • Or, when you add up the weight of all the pieces, you get the weight of the original thing. • The world is granular; it is – Sand rather than shampoo – Grapes rather than jello • The world is tinkertoys – molecules are built from atoms 02 -21 -09 17

#3 Atoms • Words that may come up. (If they don’t ask, don’t bring them up; Keep to the simple model) – Electron, proton, neutron: subatomic particles, they will be discussed as more complex MODELS – Element: a group of atoms all of which have the same number of protons – Ion: a atom in which the number of electrons is not the same as the number of protons – Isotopes: atoms that have the same number of protons but different numbers of neutrons 02 -21 -09 18

#3 Atoms • What do we need to know about atoms? – What is your weight? – What can I build with you? 02 -21 -09 19

#4 Atoms Seeing Without Seeing • The garbage bag contains models of atoms, but you may not use your eyes to see them. • Same routine as before, but B starts out as the “doer”. Put both hands in the bag. A starts out as the “recorder”. • Halfway through switch with your other team (1 – 2, 3 – 4, etc. ) 02 -21 -09 20

Atoms What data do we have? • Atomic Force Microscopy – A very sensitive probe is scanned across the surface, and the force on the probe is measured – By using electronics to keep the force constant, we can – line by line – generate a profile of the surface – The best instruments can “feel” individual atoms. – Conclusion: the world is granular. 02 -21 -09 21

Atoms What data do we have? • • • Atomic Force Microscopy (neat websites) http: //www. mee-inc. com/afm. html http: //www. rhk-tech. com/hall/Na. Cl-mica. html http: //stm 2. nrl. navy. mil/how-afm. html http: //www. omicron. de/index 2. html? /results/atomi c_resolution_on_si_111_7 x 7_in_non_contact_mo de_afm/~Omicron 02 -21 -09 22

Atoms What AFM data do we have? Silicon surface 02 -21 -09 23

Atoms What AFM data do we have? Na. Cl (salt) surface 02 -21 -09 24

Atoms What AFM data do we have? • Conclusion: – The world “feels” granular. 02 -21 -09 25

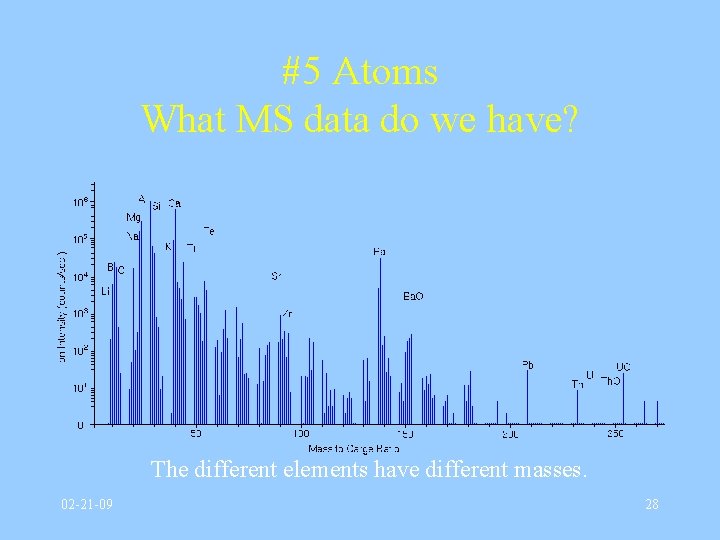
Atoms What MS data do we have? • Mass Spectrometry separates atoms (actually ions) according to their differing masses. • Different masses have different trajectories! • Real mass spectrometers require a very good vacuum, and they are expensive. 02 -21 -09 26

Atoms What MS data do we have? • Mass Spectrometry separates atoms (actually ions) according to their differing masses. • Neat websites! • http: //www. chem. arizona. edu/massspec/example_ html/examples. html • http: //www. cea. com/cai/simstheo/mspectra. htm • http: //www. chemguide. co. uk/analysis/masspec/ele ments. html 02 -21 -09 27

#5 Atoms What MS data do we have? The different elements have different masses. 02 -21 -09 28

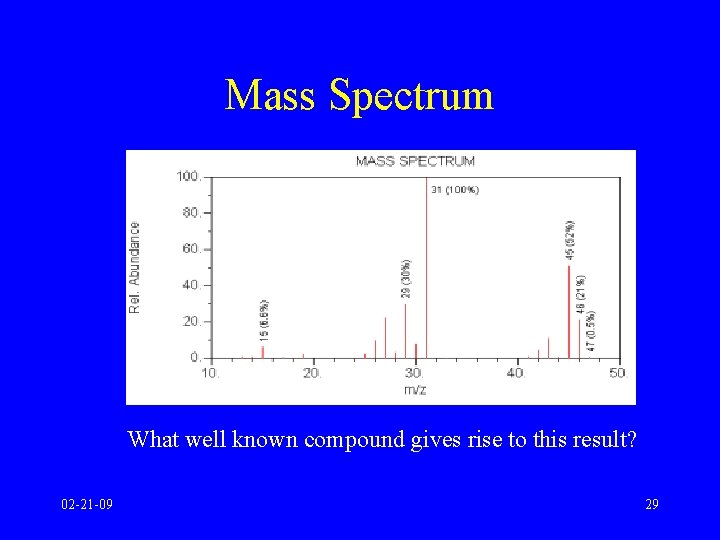
Mass Spectrum What well known compound gives rise to this result? 02 -21 -09 29

Atoms AFM and MS • AFM – The AFM box allows students to mimic the measurements made with a real AFM. Maybe you can feel individual atoms? • MS – The mass spectrometer allows students to mimic the measurements made with a real mass spectrometer. Do you want to see the trajectories of your atoms? 02 -21 -09 30

Should I use this approach in my class? • It (probably) will help students with the fundamental concepts of chemistry. • Perhaps you are constrained by the sequencing of chemistry instruction? 02 -21 -09 31

Should I use this approach in my class? • Perhaps you are constrained by the sequencing of chemistry instruction? • 8 th grade ? ? ? [pre-AP chemistry Freshman Chemistry degree in chemistry] 02 -21 -09 32

Should I use this approach in my class? • It (probably) will help students with the fundamental concepts of chemistry. • Perhaps you are constrained by TEKS and TAKS? 02 -21 -09 33

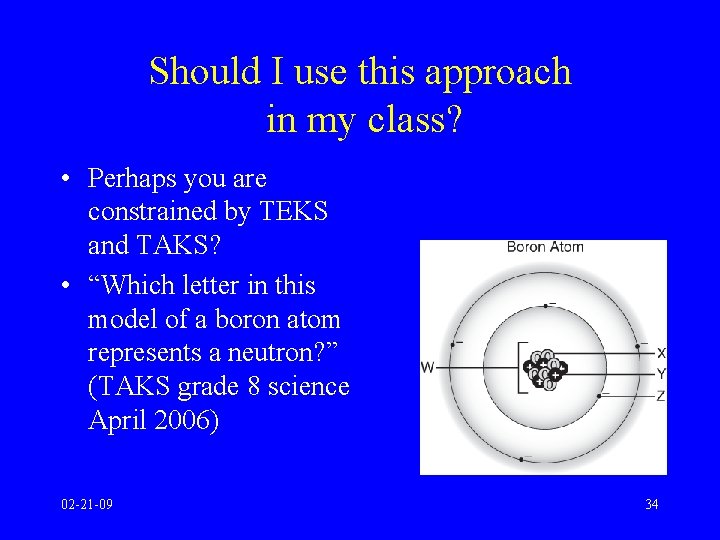
Should I use this approach in my class? • Perhaps you are constrained by TEKS and TAKS? • “Which letter in this model of a boron atom represents a neutron? ” (TAKS grade 8 science April 2006) 02 -21 -09 34
 Ron melton
Ron melton Xelpros coupon
Xelpros coupon Ron melton od
Ron melton od At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Lynn university library hours
Lynn university library hours Lynn university bookstore
Lynn university bookstore Teoria autogena
Teoria autogena Christine e lynn
Christine e lynn Lynn brewer enron
Lynn brewer enron Thorpe park aims and objectives
Thorpe park aims and objectives Jerusalem lynn
Jerusalem lynn Doru pope seattle
Doru pope seattle Lynn white jr
Lynn white jr Lynn savoie
Lynn savoie Lynn egan
Lynn egan Lynn lawrence optometry
Lynn lawrence optometry Lynn cohn mediator
Lynn cohn mediator Optical cross examples
Optical cross examples Lynn potts md
Lynn potts md Shiloh book characters
Shiloh book characters Wharton executive coaching and feedback program
Wharton executive coaching and feedback program How many siblings did loretta lynn have
How many siblings did loretta lynn have Mary lynn manns
Mary lynn manns Shamayi lynn
Shamayi lynn Till we meet again vera lynn
Till we meet again vera lynn Lynn curlee smoking angels
Lynn curlee smoking angels Lynn zechiedrich
Lynn zechiedrich Sheila ostrander
Sheila ostrander Steven benson murder
Steven benson murder Lynn luo
Lynn luo Lynn olson intel
Lynn olson intel Lynn thier
Lynn thier Mary lynn manns
Mary lynn manns Judge donnelly cook county
Judge donnelly cook county Lynn zimmerman attorney
Lynn zimmerman attorney