Atkins de Paula Chapter 4 Physical Transformations of

![Phase diagram of an organic superconductor -ET 2 Cu[N(CN)2]Cl temperature vs. pressure metal n. Phase diagram of an organic superconductor -ET 2 Cu[N(CN)2]Cl temperature vs. pressure metal n.](https://slidetodoc.com/presentation_image_h2/9f1e762a1011e73adc6dbd3a5c51bab2/image-5.jpg)



- Slides: 23

Atkins & de Paula, Chapter 4 Physical Transformations of Pure Substances Read it!

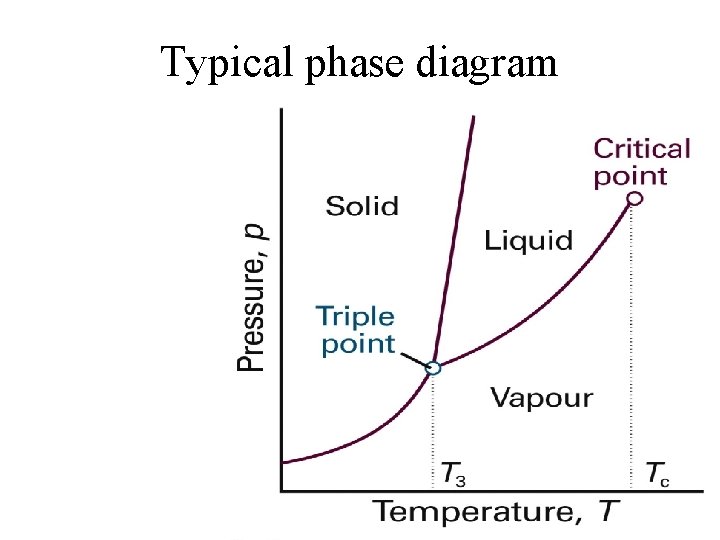
Typical phase diagram

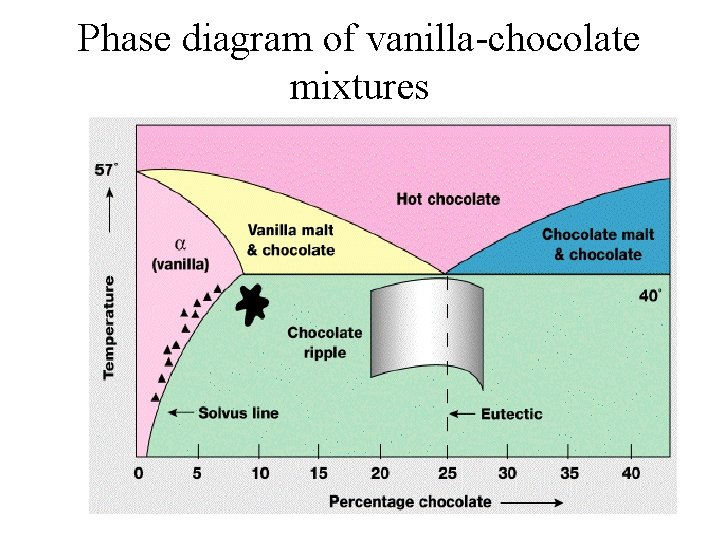
Phase diagram of vanilla-chocolate mixtures

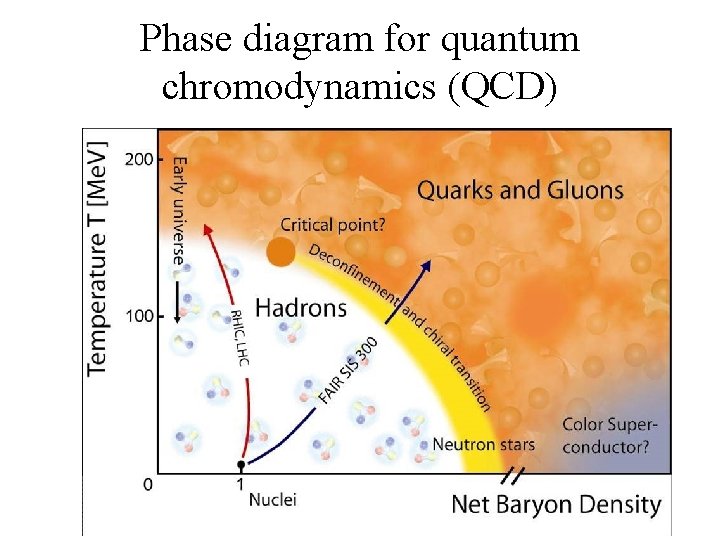
Phase diagram for quantum chromodynamics (QCD)
![Phase diagram of an organic superconductor ET 2 CuNCN2Cl temperature vs pressure metal n Phase diagram of an organic superconductor -ET 2 Cu[N(CN)2]Cl temperature vs. pressure metal n.](https://slidetodoc.com/presentation_image_h2/9f1e762a1011e73adc6dbd3a5c51bab2/image-5.jpg)
Phase diagram of an organic superconductor -ET 2 Cu[N(CN)2]Cl temperature vs. pressure metal n. b. first-order transition from Mott insulator to superconductor

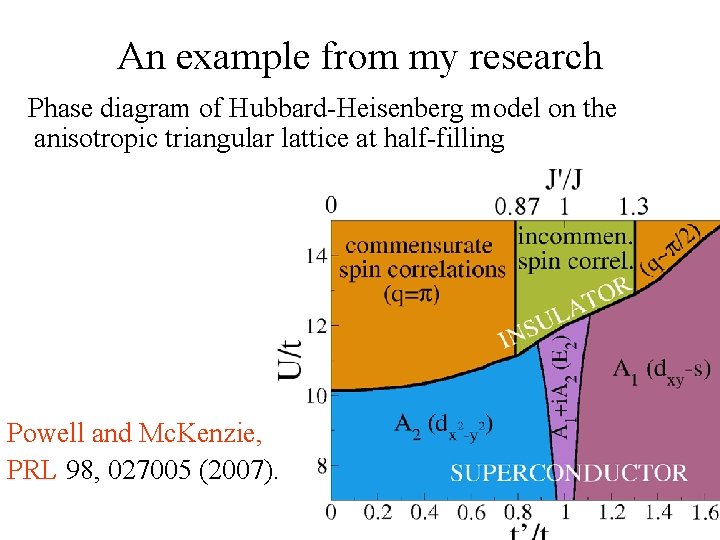
An example from my research Phase diagram of Hubbard-Heisenberg model on the anisotropic triangular lattice at half-filling Powell and Mc. Kenzie, PRL 98, 027005 (2007).

Entropy vs. Energy • Phase transitions occur because of this competition. • Generally decreasing the temperature leads to transitions into phases with lower internal energy and lower entropy.

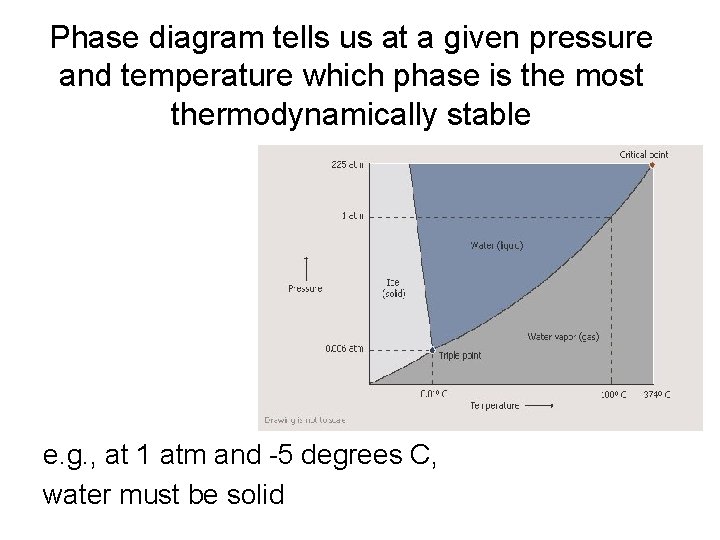
Phase diagram tells us at a given pressure and temperature which phase is the most thermodynamically stable e. g. , at 1 atm and -5 degrees C, water must be solid

Two important consequences of the second law • If at given pressure and temperature there is only one stable phase then its Gibbs free energy must be less than that of other possible phases • If two distinct phases can co-exist at a particular P, T then they must have the same Gibbs free energy.

Two phases in equilibrium

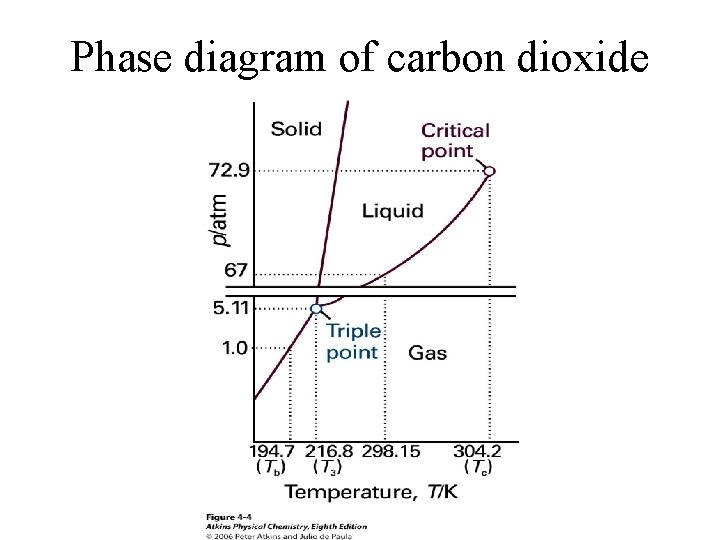
Phase diagram of carbon dioxide

Sublimation of carbon dioxide (dry ice) • . . dvd demosphase transitions1518 sublime. VOB

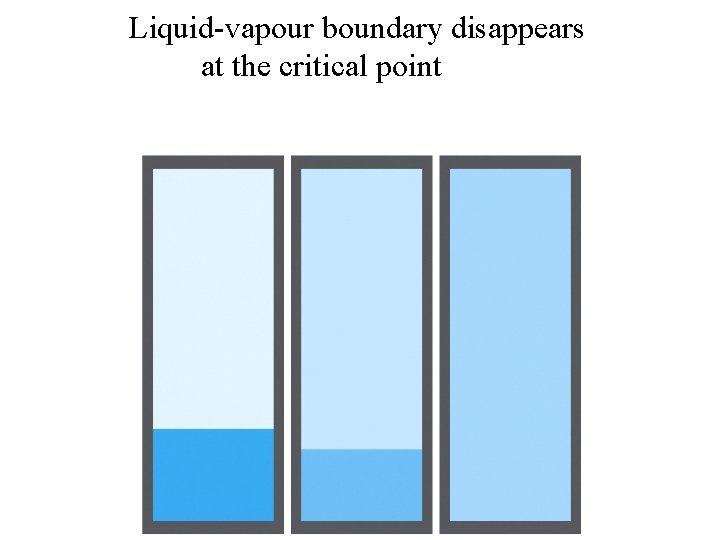
Liquid-vapour boundary disappears at the critical point

Critical point of carbon dioxide. . dvd demosphase transitions1511 critical pt. VOB

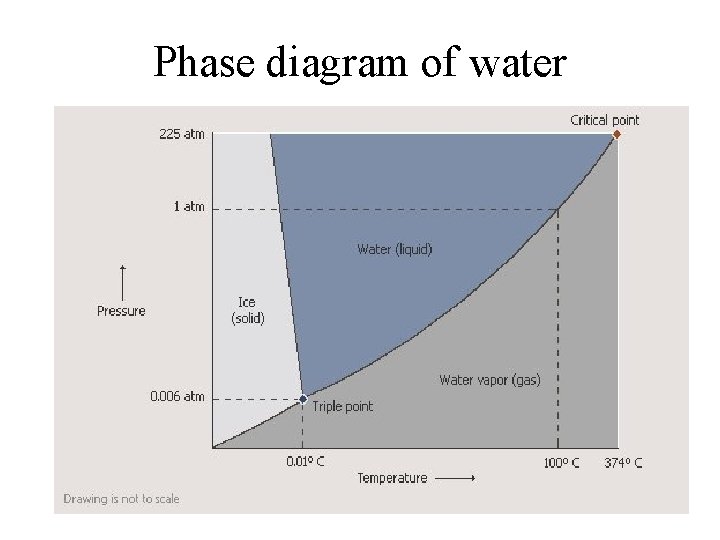
Phase diagram of water

Exercise • Compare the phase diagrams of water and carbon dioxide • Note two qualitative differences

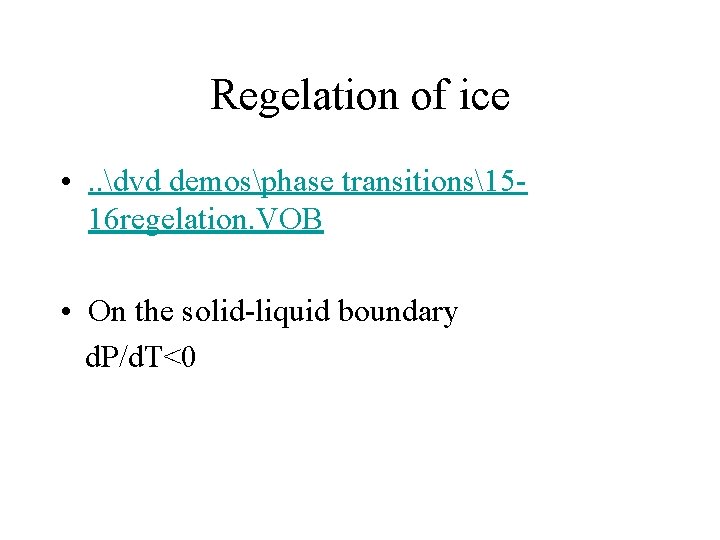
Regelation of ice • . . dvd demosphase transitions1516 regelation. VOB • On the solid-liquid boundary d. P/d. T<0

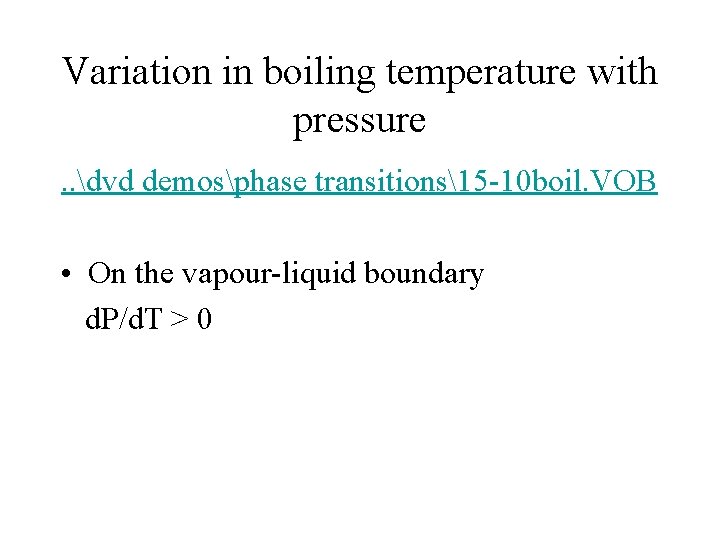
Variation in boiling temperature with pressure. . dvd demosphase transitions15 -10 boil. VOB • On the vapour-liquid boundary d. P/d. T > 0

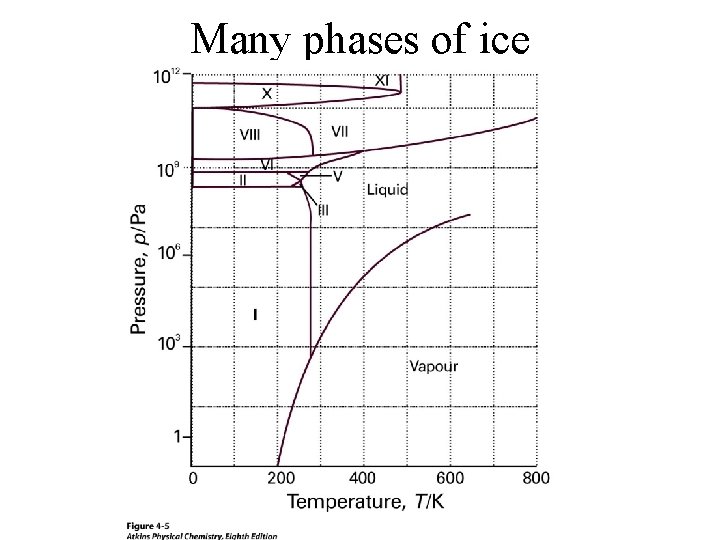
Many phases of ice

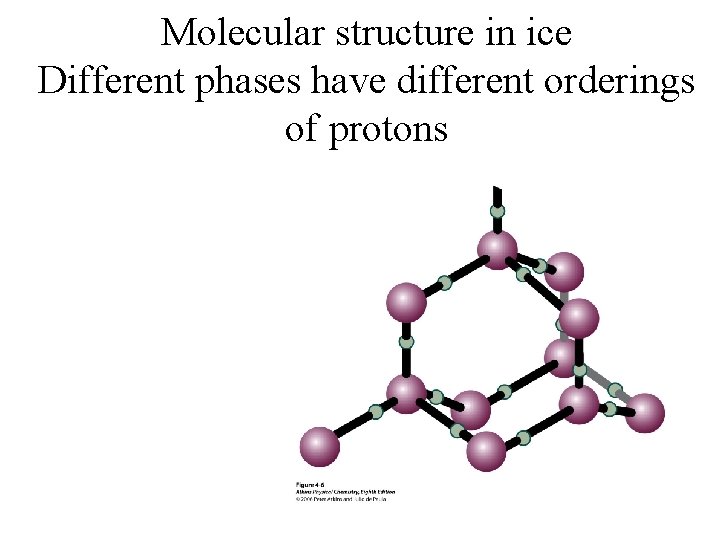
Molecular structure in ice Different phases have different orderings of protons

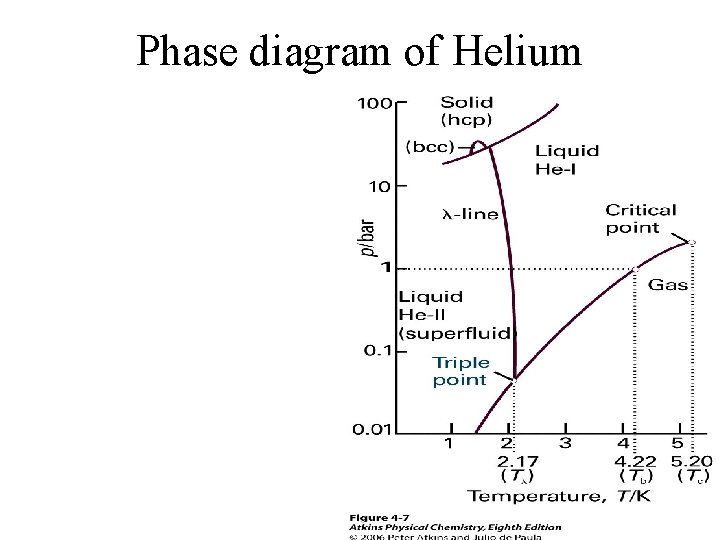
Phase diagram of Helium

Superfluids • A new state of matter • Superfluids have zero viscosity • Very large thermal conductivity • Bose-Einstein condensates

• You. Tube - Re: Superfluid helium