Chemical Kinetics Elements of Physical Chemistry Atkins de

![Rate Law How does the rate depend upon [ ]s? l Find out by Rate Law How does the rate depend upon [ ]s? l Find out by](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-4.jpg)
![Experimental rate laws? l Rate = k [CO][Cl 2]1/2 CO + Cl 2 ® Experimental rate laws? l Rate = k [CO][Cl 2]1/2 CO + Cl 2 ®](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-5.jpg)
![First-order reaction A plot of ln[A] versus t gives a straight line of slope First-order reaction A plot of ln[A] versus t gives a straight line of slope](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-8.jpg)
![A ® P assume that -(d[A]/dt) = k [A]1 10 A ® P assume that -(d[A]/dt) = k [A]1 10](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-10.jpg)
![Integrated rate equation ln [A] = -k t + ln [A]0 11 Integrated rate equation ln [A] = -k t + ln [A]0 11](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-11.jpg)
![l Half life: first-order reaction The time taken for [A] to drop to half l Half life: first-order reaction The time taken for [A] to drop to half](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-12.jpg)

![When is a reaction over? l [A] = [A]0 exp{-kt} Technically [A]=0 only after When is a reaction over? l [A] = [A]0 exp{-kt} Technically [A]=0 only after](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-14.jpg)
![Second-order reaction A plot of 1/[A] versus t gives a straight line of slope Second-order reaction A plot of 1/[A] versus t gives a straight line of slope](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-16.jpg)

![Molecularity of elementary reactions? l l 20 Unimolecular (decay) A ® P - (d[A]/dt) Molecularity of elementary reactions? l l 20 Unimolecular (decay) A ® P - (d[A]/dt)](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-20.jpg)
![CO + Cl 2 l ´ Exptal rate law: COCl 2 - (d[CO]/dt) = CO + Cl 2 l ´ Exptal rate law: COCl 2 - (d[CO]/dt) =](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-21.jpg)
![- (d[CO]/dt) = k 2 [Cl] [CO] If steps 2 & 3 are slow - (d[CO]/dt) = k 2 [Cl] [CO] If steps 2 & 3 are slow](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-22.jpg)
![H 2 + I 2 2 HI l l Predict: + (1/2) (d[HI]/dt) = H 2 + I 2 2 HI l l Predict: + (1/2) (d[HI]/dt) =](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-23.jpg)

![Flowvs batch reactors [A]=[A]0 exp{-kt} Stirred flow; perfect mixing l spherical shape of volume Flowvs batch reactors [A]=[A]0 exp{-kt} Stirred flow; perfect mixing l spherical shape of volume](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-27.jpg)












- Slides: 52

Chemical Kinetics – ‘Elements of Physical Chemistry’, Atkins & de Paula – “Reaction Kinetics” , Pilling & Seakins – “An Examples Course in CK” , Campbell MRapid revision of second year MKinetics in flow reactors; plug vs stirred MTemperature dependence; Arrhenius equation MComplex reactions – chain reactions – polymerisations – explosions; branching chain reactions 1

Revision of 2 nd year l 5 Br- + Br. O 3 - + 6 H+ = 3 Br 2 + 3 H 2 O Rate? conc/time or in SI mol dm-3 s-1 – (1/5)(d[Br-]/dt) = – (1/6) (d[H+]/dt) = (1/3)(d[Br 2]/dt) = (1/3)(d[H 2 O]/dt) Rate law? Comes from experiment l Rate = k [Br-][Br. O 3 -][H+]2 where k is the rate constant (variable units) 2

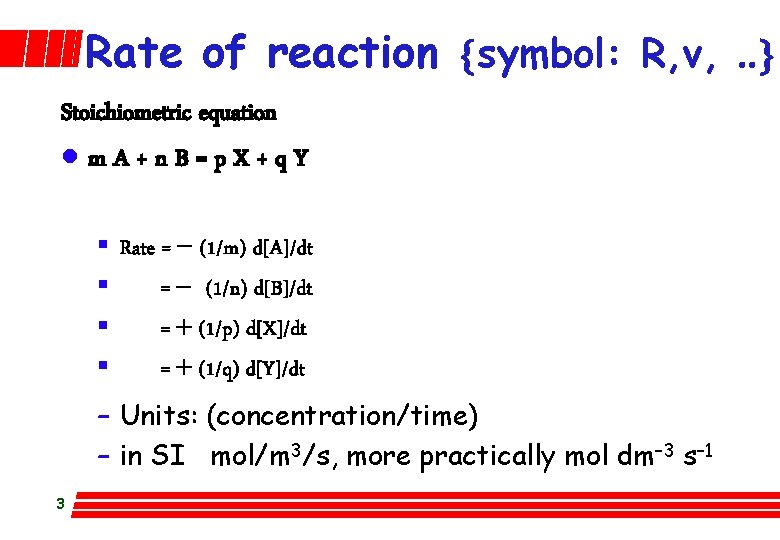
Rate of reaction {symbol: R, v, . . } Stoichiometric equation lm. A+n. B=p. X+q. Y § Rate = - (1/m) d[A]/dt § = - (1/n) d[B]/dt § = + (1/p) d[X]/dt § = + (1/q) d[Y]/dt – Units: (concentration/time) – in SI mol/m 3/s, more practically mol dm– 3 s– 1 3
![Rate Law How does the rate depend upon s l Find out by Rate Law How does the rate depend upon [ ]s? l Find out by](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-4.jpg)
Rate Law How does the rate depend upon [ ]s? l Find out by experiment l The Rate Law equation l R = kn [A]a [B]b … (for many reactions) – order, n = a + b + … (dimensionless) – rate constant, kn (units depend on n) – Rate = kn when each [conc] = unity 4
![Experimental rate laws l Rate k COCl 212 CO Cl 2 Experimental rate laws? l Rate = k [CO][Cl 2]1/2 CO + Cl 2 ®](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-5.jpg)
Experimental rate laws? l Rate = k [CO][Cl 2]1/2 CO + Cl 2 ® COCl 2 – Order = 1. 5 or one-and-a-half order l Rate = k [H 2][I 2] H 2 + I 2 ® 2 HI – Order = 2 or second order l H 2 + Br 2 ® 2 HBr Rate = k [H 2][Br 2] / (1 + k’ {[HBr]/[Br 2]} ) – Order = undefined or none 5

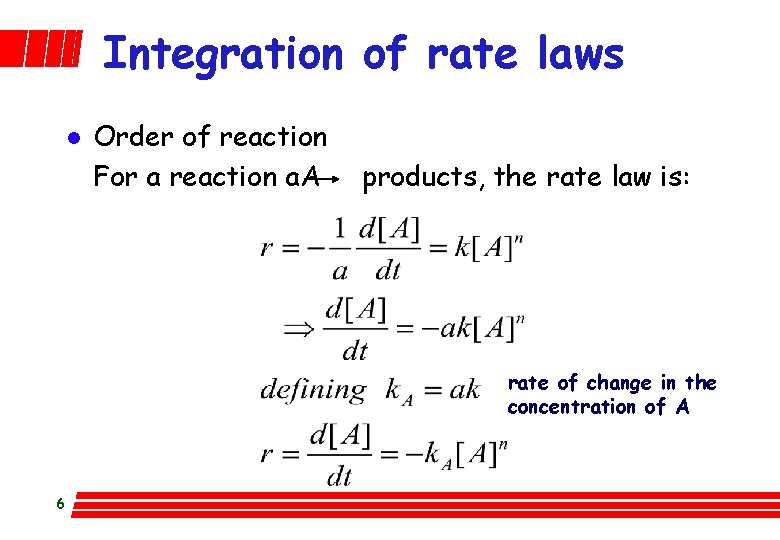
Integration of rate laws l Order of reaction For a reaction a. A products, the rate law is: rate of change in the concentration of A 6

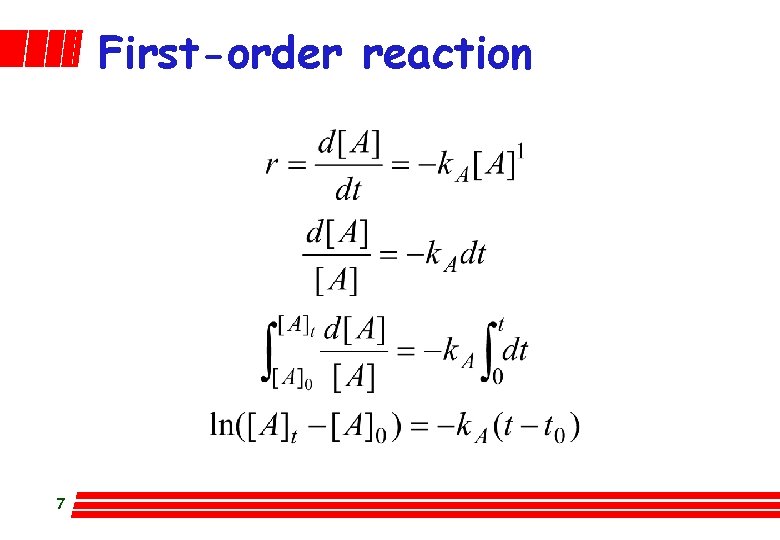
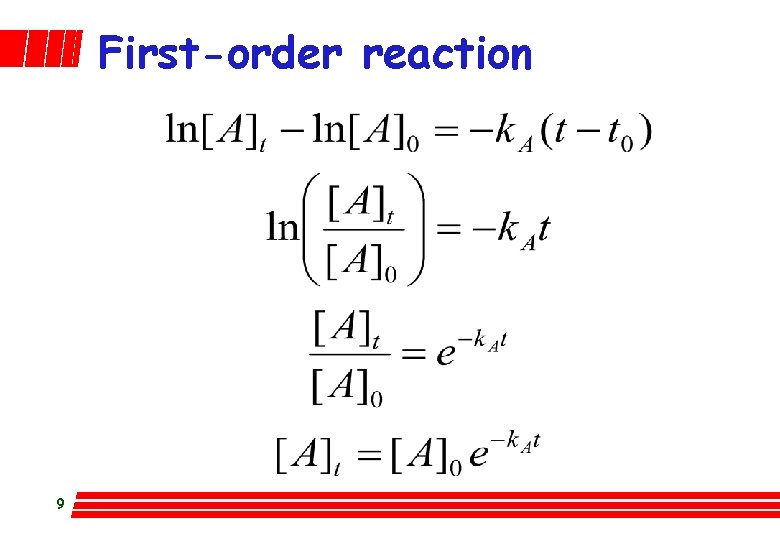
First-order reaction 7
![Firstorder reaction A plot of lnA versus t gives a straight line of slope First-order reaction A plot of ln[A] versus t gives a straight line of slope](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-8.jpg)
First-order reaction A plot of ln[A] versus t gives a straight line of slope -k. A if r = k. A[A]1 8

First-order reaction 9
![A P assume that dAdt k A1 10 A ® P assume that -(d[A]/dt) = k [A]1 10](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-10.jpg)
A ® P assume that -(d[A]/dt) = k [A]1 10
![Integrated rate equation ln A k t ln A0 11 Integrated rate equation ln [A] = -k t + ln [A]0 11](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-11.jpg)
Integrated rate equation ln [A] = -k t + ln [A]0 11
![l Half life firstorder reaction The time taken for A to drop to half l Half life: first-order reaction The time taken for [A] to drop to half](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-12.jpg)
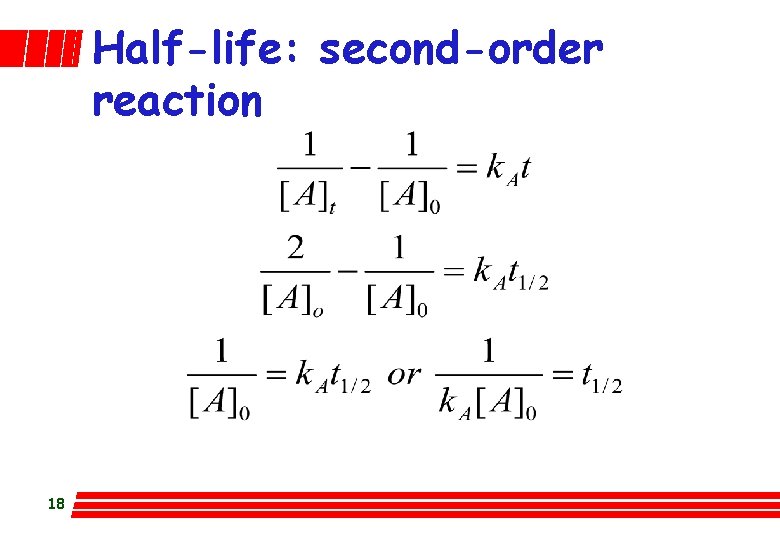
l Half life: first-order reaction The time taken for [A] to drop to half its original value is called the reaction’s half-life, t 1/2. Setting [A] = ½[A]0 and t = t 1/2 in: 12

Half life: first-order reaction 13
![When is a reaction over l A A0 expkt Technically A0 only after When is a reaction over? l [A] = [A]0 exp{-kt} Technically [A]=0 only after](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-14.jpg)
When is a reaction over? l [A] = [A]0 exp{-kt} Technically [A]=0 only after infinite time 14

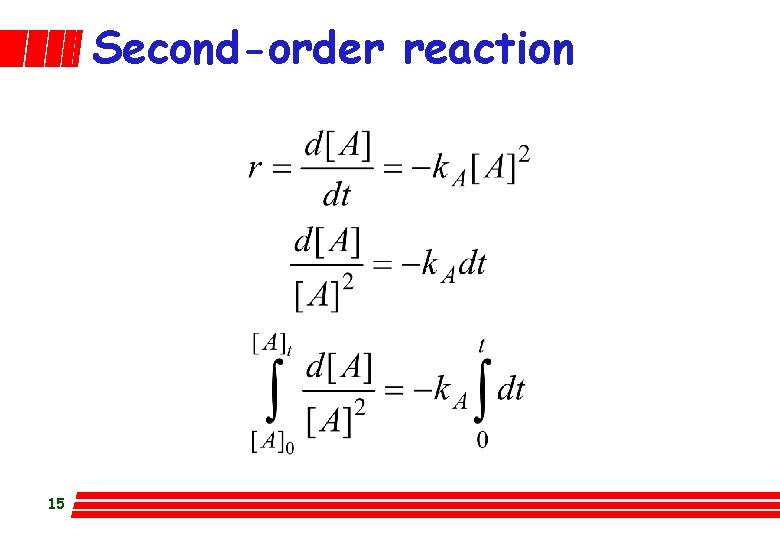
Second-order reaction 15
![Secondorder reaction A plot of 1A versus t gives a straight line of slope Second-order reaction A plot of 1/[A] versus t gives a straight line of slope](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-16.jpg)
Second-order reaction A plot of 1/[A] versus t gives a straight line of slope k. A if r = k. A[A]2 16

Second order test: A + A ® P 17

Half-life: second-order reaction 18

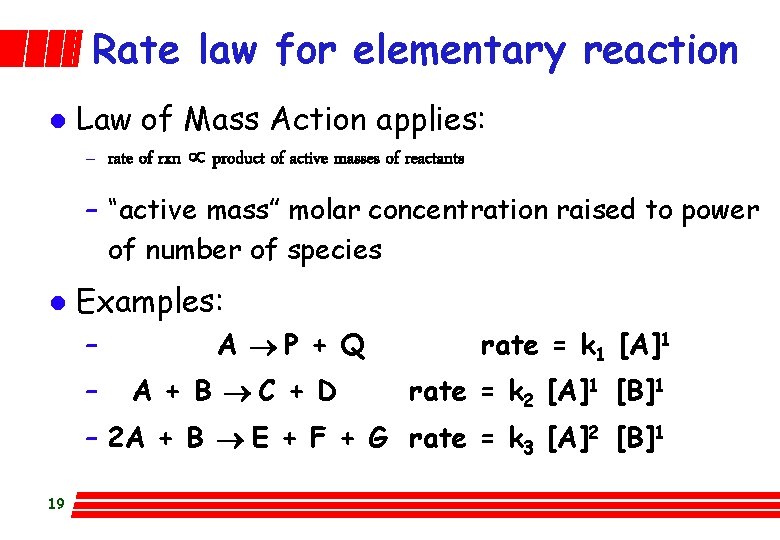
Rate law for elementary reaction l Law of Mass Action applies: – rate of rxn µ product of active masses of reactants – “active mass” molar concentration raised to power of number of species l Examples: – – A P + Q A + B C + D rate = k 1 [A]1 rate = k 2 [A]1 [B]1 – 2 A + B E + F + G rate = k 3 [A]2 [B]1 19
![Molecularity of elementary reactions l l 20 Unimolecular decay A P dAdt Molecularity of elementary reactions? l l 20 Unimolecular (decay) A ® P - (d[A]/dt)](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-20.jpg)
Molecularity of elementary reactions? l l 20 Unimolecular (decay) A ® P - (d[A]/dt) = k 1 [A] Bimolecular (collision) A + B ® P - (d[A]/dt) = k 2 [A] [B] Termolecular (collision) A + B + C ® P - (d[A]/dt) = k 3 [A] [B] [C] No other are feasible! Statistically highly unlikely.
![CO Cl 2 l Exptal rate law COCl 2 dCOdt CO + Cl 2 l ´ Exptal rate law: COCl 2 - (d[CO]/dt) =](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-21.jpg)
CO + Cl 2 l ´ Exptal rate law: COCl 2 - (d[CO]/dt) = k [CO] [Cl 2]1/2 – Conclusion? : reaction does not proceed as written – “Elementary” reactions; rxns. that proceed as written at the molecular level. Cl 2 Cl + Cl Cl + CO COCl + Cl 2 COCl 2 + Cl (3) Cl + Cl 2 l l (1) (2) (4) u decay u collisional – Steps 1 thru 4 comprise the “mechanism” of the reaction. 21
![dCOdt k 2 Cl CO If steps 2 3 are slow - (d[CO]/dt) = k 2 [Cl] [CO] If steps 2 & 3 are slow](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-22.jpg)
- (d[CO]/dt) = k 2 [Cl] [CO] If steps 2 & 3 are slow in comparison to 1 & 4 then, Cl 2 ⇌ 2 Cl or K = [Cl]2 / [Cl 2] So [Cl] = ÖK × [Cl 2]1/2 Hence: l - (d[CO] / dt) = k 2 × ÖK × [CO][Cl 2]1/2 Predict that: observed k = k 2 × ÖK l 22 Therefore mechanism confirmed (? )
![H 2 I 2 2 HI l l Predict 12 dHIdt H 2 + I 2 2 HI l l Predict: + (1/2) (d[HI]/dt) =](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-23.jpg)
H 2 + I 2 2 HI l l Predict: + (1/2) (d[HI]/dt) = k [H 2] [I 2] But if via: – I 2 2 I – I + H 2 2 HI – I + I I 2 rate = k 2 [I]2 [H 2] Assume, as before, that 1 & 3 are fast cf. to 2 Then: I 2 ⇌ 2 I or K = [I]2 / [I 2] l Rate = k 2 [I]2 [H 2] = k 2 K [I 2] [H 2] (identical) Check? I 2 + hn ® 2 I (light of 578 nm) 23

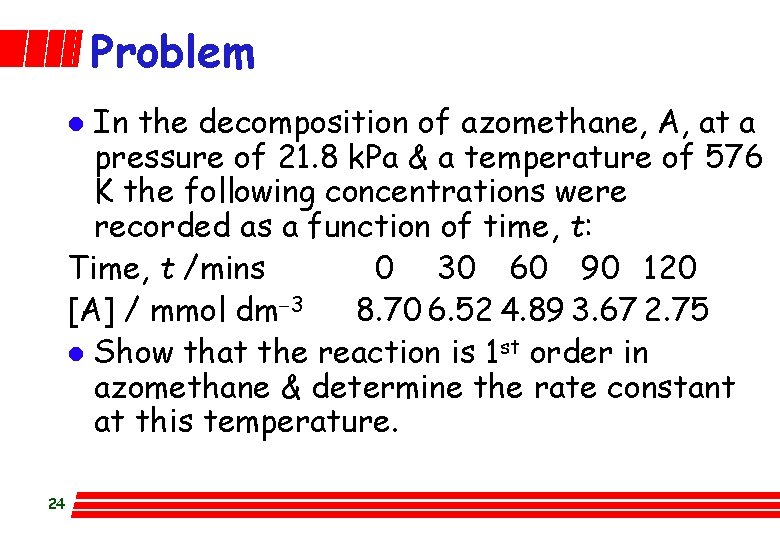
Problem In the decomposition of azomethane, A, at a pressure of 21. 8 k. Pa & a temperature of 576 K the following concentrations were recorded as a function of time, t: Time, t /mins 0 30 60 90 120 [A] / mmol dm-3 8. 70 6. 52 4. 89 3. 67 2. 75 l Show that the reaction is 1 st order in azomethane & determine the rate constant at this temperature. l 24

Recognise that this is a rate law question dealing with the integral method. - (d[A]/dt) = k [A]? = k [A]1 Re-arrange & integrate (bookwork) l Test: ln [A] = - k t + ln [A]0 Complete table: Time, t /mins 0 30 60 90 120 ln [A] 2. 16 1. 88 1. 59 1. 30 1. 01 l Plot ln [A] along y-axis; t along x-axis l Is it linear? Yes. Conclusion follows. Calc. slope as: -0. 00959 so k = + 9. 6´ 10 -3 min-1 25

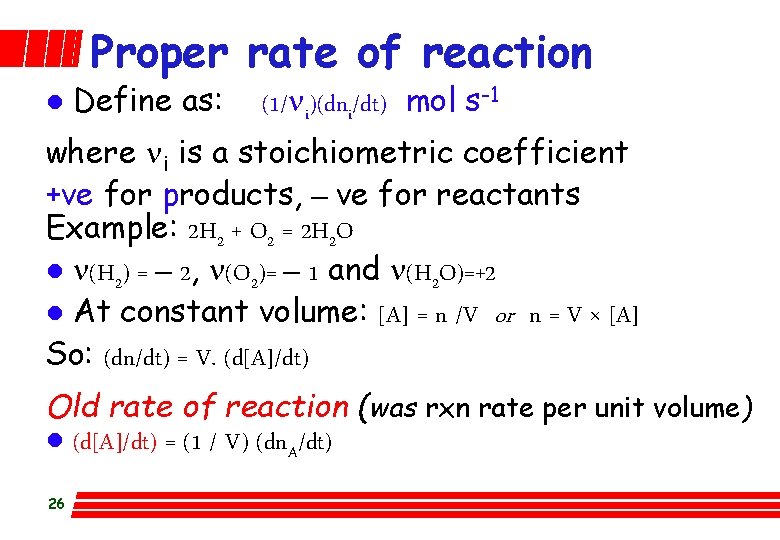
Proper rate of reaction l Define as: (1/ni)(dni/dt) mol s-1 where ni is a stoichiometric coefficient +ve for products, – ve for reactants Example: 2 H 2 + O 2 = 2 H 2 O l n(H 2) = – 2, n(O 2)= – 1 and n(H 2 O)=+2 l At constant volume: [A] = n /V or n = V × [A] So: (dn/dt) = V. (d[A]/dt) Old rate of reaction (was rxn rate per unit volume) l (d[A]/dt) = (1 / V) (dn. A/dt) 26
![Flowvs batch reactors AA0 expkt Stirred flow perfect mixing l spherical shape of volume Flowvs batch reactors [A]=[A]0 exp{-kt} Stirred flow; perfect mixing l spherical shape of volume](https://slidetodoc.com/presentation_image/2d5c18e61a4f9893429602c769cedbb4/image-27.jpg)
Flowvs batch reactors [A]=[A]0 exp{-kt} Stirred flow; perfect mixing l spherical shape of volume V [A]outlet = [A]inlet / { 1 + k (V/n)} ‘Plug’ flow; no mixing at all l cylindrical shape of volume V [A]outlet = [A]inlet exp{ - k (V/n)} NB (V/n) has dimensions (m 3 / m 3 s-1) = s A ‘residence’ or ‘contact’ time 27

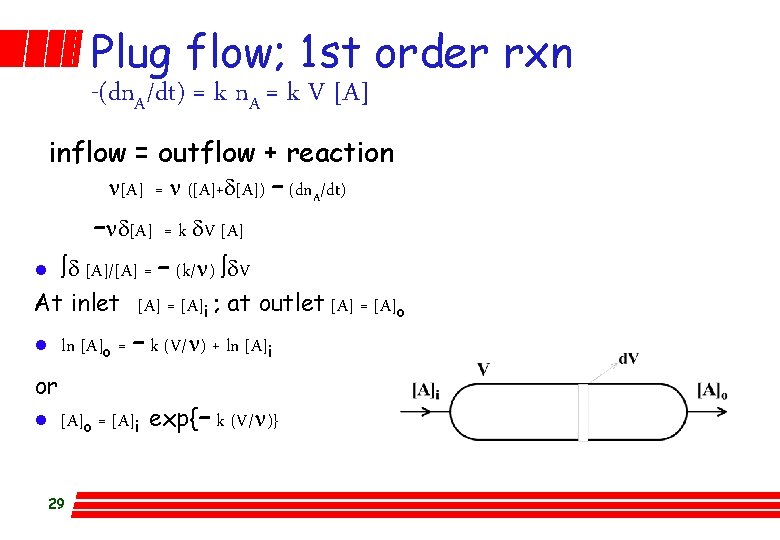
Stirred flow; 1 st order -(dn. A/dt) = k n. A = k V [A] l inflow = outflow + reaction Assume system at constant volume so that inlet flow rate is equal to outlet flow rate l n[A]i = n[A]o - (dn. A/dt) l n[A]i = n[A]o + k V [A]o l [A]o = [A]i / {1 + k(V/n)} l Test? vary v, measure [A]o & [A]i, is k the same? 28

Plug flow; 1 st order rxn -(dn. A/dt) = k n. A = k V [A] inflow = outflow + reaction n[A] = n ([A]+d[A]) – (dn. A/dt) –nd[A] = k d. V [A] l òd [A]/[A] = – (k/n) òd. V At inlet [A] = [A]i ; at outlet [A] = [A]o l or l ln [A]o = – k (V/n) + ln [A]i [A]o = [A]i exp{– k (V/n)} 29

Flow reactor problem l Peracetic acid vapour at 2% by volume in nitrogen carrier gas at a total pressure of 101 k. Pa and 490 K flows thru 1. 4 mm radius teflon tubing and is sampled 1. 2 m downstream with the following results: ([A]o/[A]i) l 0. 346 0. 578 0. 718 n / m 3 s-1 3. 71´ 10 -5 7. 20´ 10 -5 11. 9´ 10 -5 Show that rxn follows 1 st order kinetics & calculate k. Eqn? [A]o=[A]i exp{- k (V/n)} or ln{[A]o/[A]i}= - k(V/n) l Reactor vol. V = pr 2 h = 3. 14 ´ (0. 0014)2 ´ 1. 2 = 7. 39 ´ 10 -6 m 3 l k = - (n / V) ´ ln{[A]o/[A]i} = 5. 3208, 5. 3410, 5. 3349 s-1 30

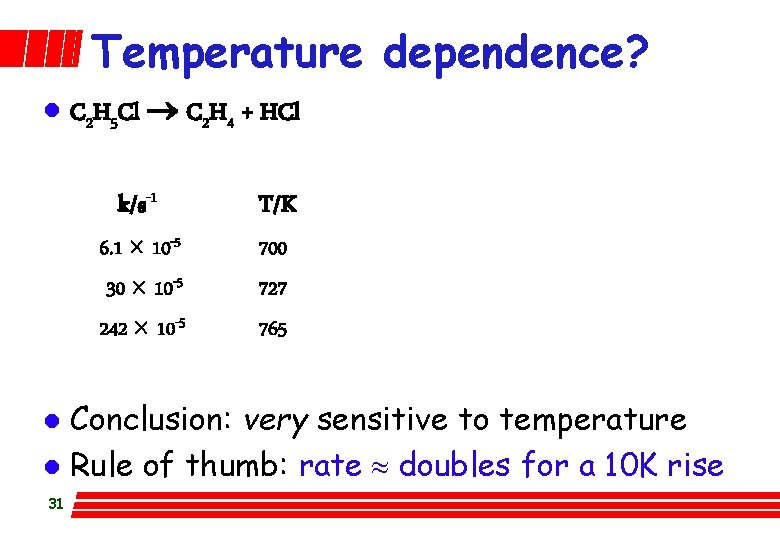
Temperature dependence? l C 2 H 5 Cl C 2 H 4 + HCl k/s-1 6. 1 ´ 10 -5 30 ´ 10 -5 242 ´ 10 -5 T/K 700 727 765 Conclusion: very sensitive to temperature l Rule of thumb: rate » doubles for a 10 K rise l 31

Details of T dependence Hood l k = A exp{ -B/T } Arrhenius l k = A exp{ - E / RT } A A-factor or pre-exponential factor º k at T ¥ E activation energy (energy barrier) J mol R gas constant. 32 -1 or k. J mol-1

Arrhenius eqn. k=A exp{-E/RT} Useful linear form: ln k = -(E/R)(1/T) + ln A l Plot ln k along Y-axis vs (1/T) along X-axis Slope is negative -(E/R); intercept = ln A l Experimental Es range from 0 to +400 k. J mol-1 Examples: – – 33 H· + HCl ® H 2 + Cl· H· + HF ® H 2 + F· C 2 H 5 I ® C 2 H 4 + HI C 2 H 6 2 CH 3 19 k. J mol-1 139 k. J mol-1 209 k. J mol-1 368 k. J mol-1

Practical Arrhenius plot, 34 origin not included

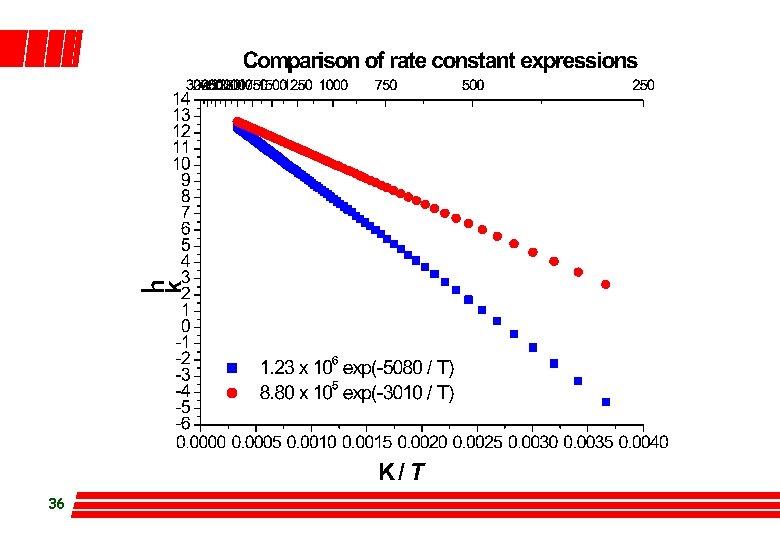
Problem l In consecutive rxns the slower step usually determines the overall rate of rxn. Diethyl adipate (DA) is hydrolysed in 2 steps as: DA ® B ® P l 35 The Arrhenius equations are: first step 1. 23´ 106 exp{-5, 080/T} s-1 second step 8. 80´ 105 exp{-3, 010/T} s-1 Which step is rate determining if the rxn is carried out in aqueous solution under atmospheric pressure?

36

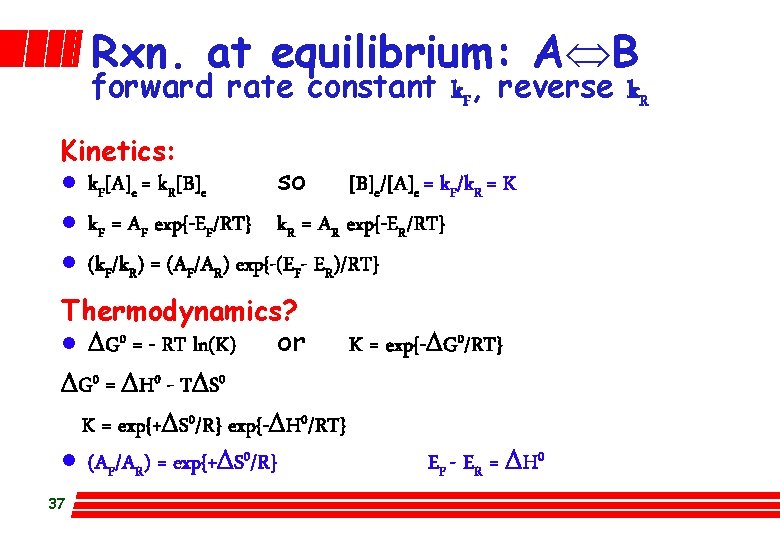
Rxn. at equilibrium: AÛB forward rate constant k. F, reverse k. R Kinetics: l k. F[A]e = k. R[B]e so [B]e/[A]e = k. F/k. R = K l k. F = AF exp{-EF/RT} k. R = AR exp{-ER/RT} l (k. F/k. R) = (AF/AR) exp{-(EF- ER)/RT} Thermodynamics? l DG 0 = - RT ln(K) or K = exp{-DG 0/RT} DG 0 = DH 0 - TDS 0 K = exp{+DS 0/R} exp{-DH 0/RT} l (AF/AR) = exp{+DS 0/R} EF - ER = DH 0 37

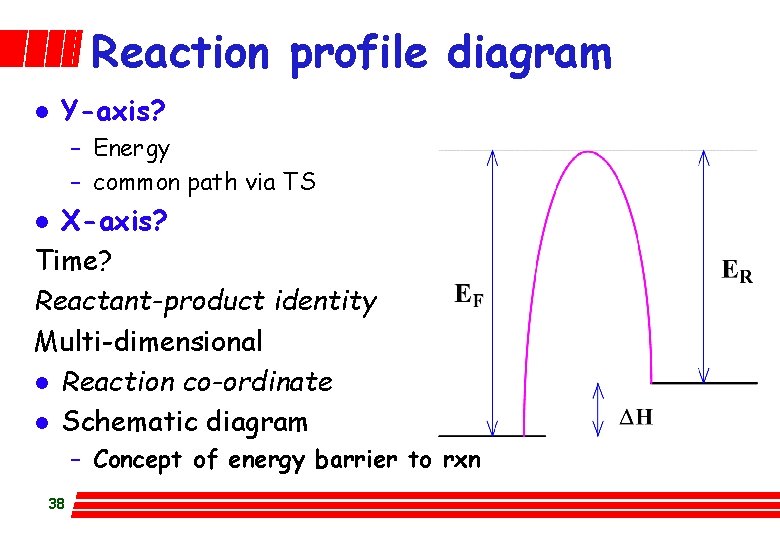
Reaction profile diagram l Y-axis? – Energy – common path via TS X-axis? Time? Reactant-product identity Multi-dimensional l Reaction co-ordinate l Schematic diagram l – Concept of energy barrier to rxn 38

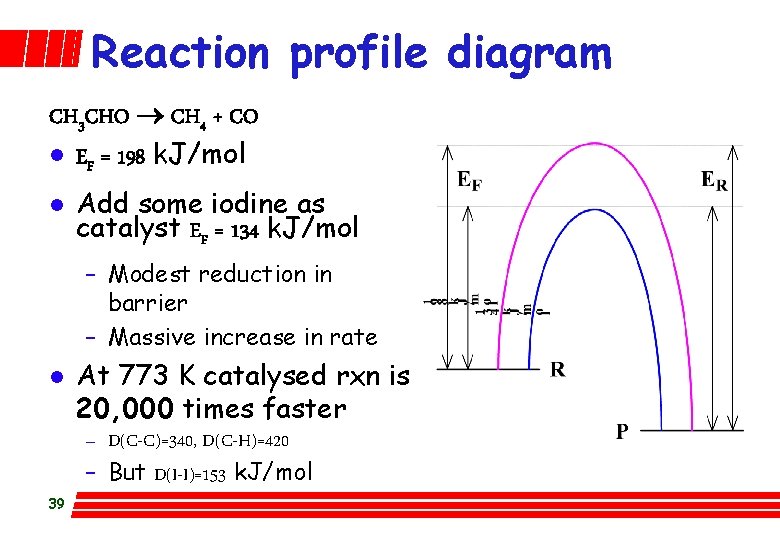
Reaction profile diagram CH 3 CHO CH 4 + CO l EF = 198 k. J/mol l Add some iodine as catalyst EF = 134 k. J/mol – Modest reduction in barrier – Massive increase in rate l 39 At 773 K catalysed rxn is 20, 000 times faster – D(C-C)=340, D(C-H)=420 – But D(I-I)=153 k. J/mol

Thermochemistry from kinetics C 2 H 6 2 CH 3· 368 k. J mol-1 EF = 368 k. J mol-1 ER = 0 k. J mol-1 DHRXN = EF-ER = + 368 k. J mol-1 = 2 DHf(CH 3·) - DHf(C 2 H 6) But DHf(C 2 H 6) = - 85 k. J mol-1 l DHf(CH 3·) = (368 -85)/2 = +141 k. J mol-1 l DHRXN = Energy of bond broken = D(H 3 C-CH 3) l 40 Þ Tables of bond dissociation energies

Problem For the rxn trans ® cis perfluorobut-2 -ene l measured k. F=3. 16´ 1013 exp{-31, 030/T} s-1 l l l Given that DH 0=+3. 42 k. J mol-1 and DS 0=-2. 03 J K-1 mol-1 Calculate k. R at 750 K. (AF/AR) = exp{+DS 0/R} Þ AR = AF exp{-DS 0/R} Þ ER = EF - DH 0 -(EF/R) = -31, 030 Þ EF = 8. 3143´ 31, 030 J mol-1 EF = 258. 0 k. J mol-1 Þ ER = 254. 6 k. J mol-1 EF - ER = DH 0 41

Common mathematical functions Kinetics, Arrhenius l k = A exp{-E/RT} Vapour pressure, Clausius-Clayperon l p = p¥ exp{-DHV/RT} Viscosity, Andrade l h = A exp{+E/RT} l 42 But re-define as inverse: F = F¥ exp{-E/RT}

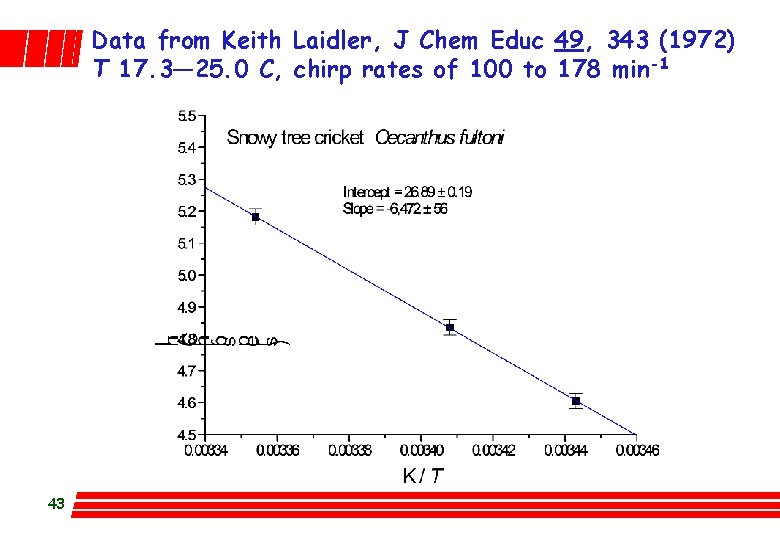
Data from Keith Laidler, J Chem Educ 49, 343 (1972) T 17. 3— 25. 0 C, chirp rates of 100 to 178 min-1 43

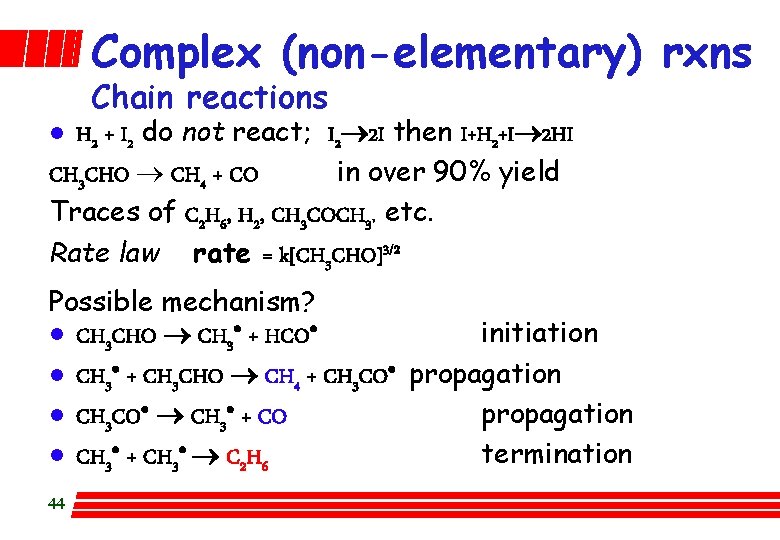
Complex (non-elementary) rxns Chain reactions H 2 + I 2 do not react; I 2 2 I then I+H 2+I 2 HI CH 3 CHO ® CH 4 + CO in over 90% yield Traces of C 2 H 6, H 2, CH 3 COCH 3, etc. Rate law rate = k[CH 3 CHO]3/2 l Possible mechanism? l CH 3 CHO CH 3· + HCO· initiation l CH 3· + CH 3 CHO CH 4 + CH 3 CO· propagation l CH 3 CO· CH 3· + CO propagation l CH 3· + CH 3· C 2 H 6 termination 44

Kinetic analysis? Mathematically difficult Steady-state approximation l l l – For reactive intermediates assume that rate of formation » rate of destruction ie (d[R]/dt) » 0 (d[CH 3·]/dt)= v 1 + v 3 - v 2 - v 4 » 0 (d[CH 3 CO·]/dt)= v 2 - v 3 » 0 Þ v 2= v 3 and v 1 = v 4 k 1[CH 3 CHO] = 2 k 4[CH 3·]2 S-S conc. [CH 3·]=(2 k 1/k 4)1/2 [CH 3 CHO]1/2 (d[CH 4]/dt) = k 2[CH 3·][CH 3 CHO] = k 2(2 k 1/k 4)1/2 [CH 3 CHO]3/2 l So kobs = k 2(2 k 1/k 4)1/2 45

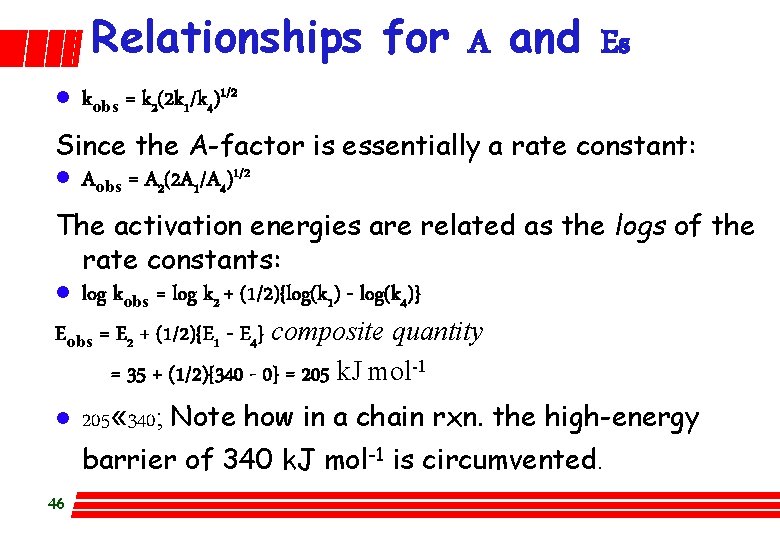
Relationships for A and Es l kobs = k 2(2 k 1/k 4)1/2 Since the A-factor is essentially a rate constant: l Aobs = A 2(2 A 1/A 4)1/2 The activation energies are related as the logs of the rate constants: l log kobs = log k 2 + (1/2){log(k 1) - log(k 4)} Eobs = E 2 + (1/2){E 1 - E 4} composite quantity = 35 + (1/2){340 - 0} = 205 k. J mol-1 l 205 « 340; Note how in a chain rxn. the high-energy barrier of 340 k. J mol-1 is circumvented. 46

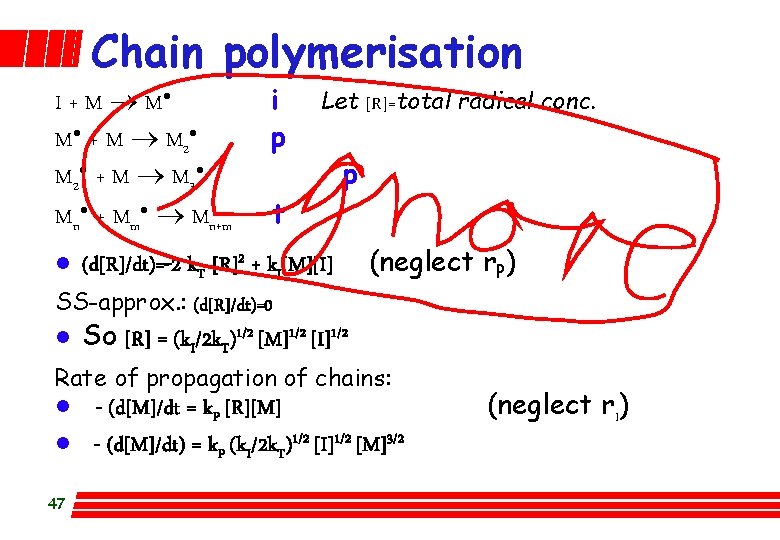
Chain polymerisation I + M ® M· M · + M ® M 2· + M ® M 3· Mn· + Mm· ® Mn+m l i p Let [R]=total radical conc. p t (d[R]/dt)=-2 k. T [R]2 + k. I[M][I] (neglect r. P) SS-approx. : (d[R]/dt)=0 l So [R] = (k. I/2 k. T)1/2 [M]1/2 [I]1/2 Rate of propagation of chains: l l 47 - (d[M]/dt = k. P [R][M] - (d[M]/dt) = k. P (k. I/2 k. T)1/2 [I]1/2 [M]3/2 (neglect r. I)

Branching chain reactions l “On a windy Thursday in the first week of May in 1937, the Hindenberg airship exploded as it approached its mooring in New Jersey, USA. Newsreel film of the disaster, in which 35 passengers & 1 ground crew died, shows that all 200, 000 m 3 of H 2 in the ship burned in under 45 s. ” – Physical Chemistry, Winn, p. 1016 – http: //www. otr. com/hindenburg. html 48

Branching chain reactions O· + H 2 ® HO· + H· The power of two l 1® 2® 4® 8® 16® 32® 64® 128® 256® 512 etc l Fifty doublings or 250 » 1015 € 1015 for the 380 million citizens of the EU l – € 2. 6 million each l 49 So reactions in which branching is important can proceed explosively

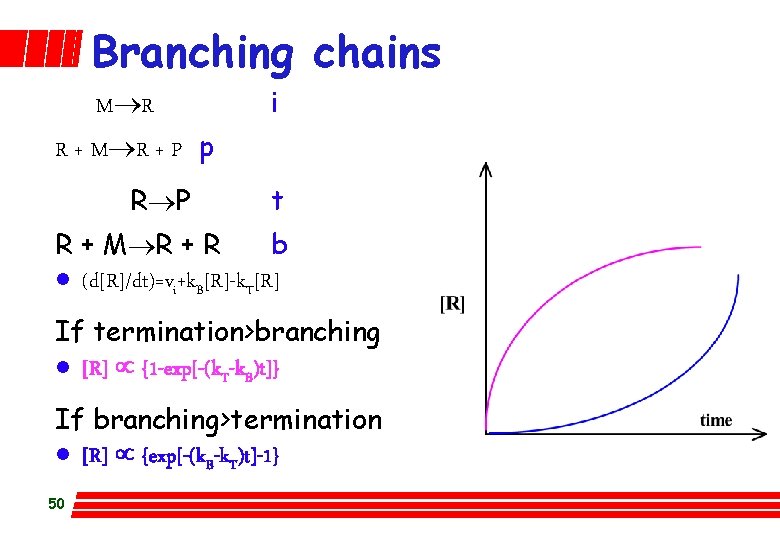
Branching chains M®R i R + M®R + P p R®P t R + M®R + R b l (d[R]/dt)=vi+k. B[R]-k. T[R] If termination>branching l [R] µ {1 -exp[-(k. T-k. B)t]} If branching>termination l [R] µ {exp[-(k. B-k. T)t]-1} 50

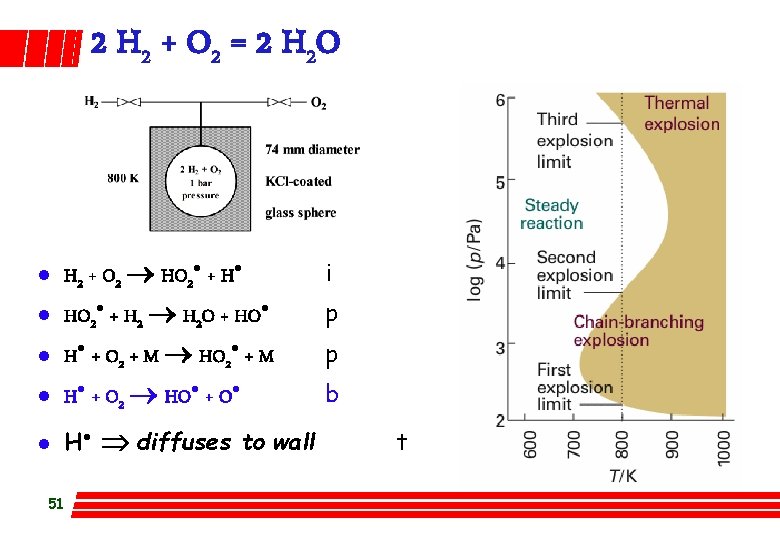
2 H 2 + O 2 = 2 H 2 O l H 2 + O 2 HO 2· + H· i H· + O 2 + M HO 2· + M p HO 2· + H 2 O + HO· p l H· + O 2 HO· + O· b l H· Þ diffuses to wall l l 51 t

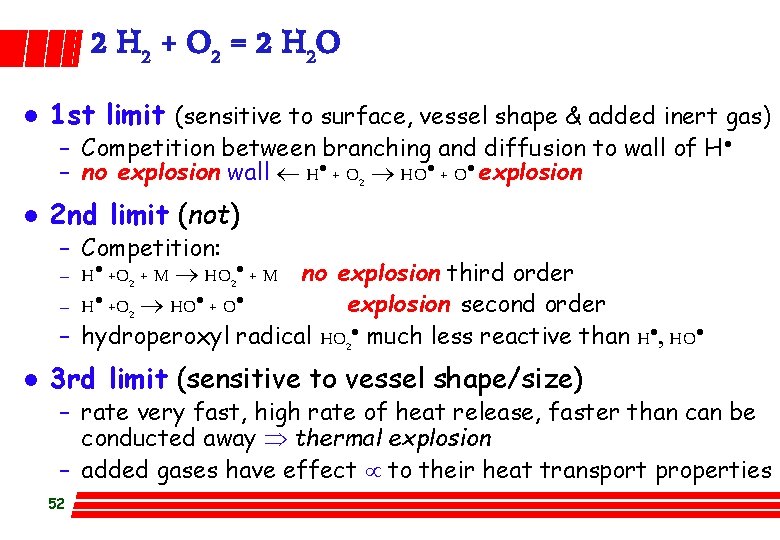
2 H 2 + O 2 = 2 H 2 O l 1 st limit (sensitive to surface, vessel shape & added inert gas) – Competition between branching and diffusion to wall of H · – no explosion wall ¬ H· + O 2 ® HO· + O· explosion l 2 nd limit (not) – – l Competition: H· +O 2 + M ® HO 2· + M no explosion third order H· +O 2 ® HO· + O· explosion second order hydroperoxyl radical HO 2· much less reactive than H·, HO· 3 rd limit (sensitive to vessel shape/size) – rate very fast, high rate of heat release, faster than can be conducted away Þ thermal explosion – added gases have effect µ to their heat transport properties 52
 Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Grade 11 chemistry unit 4
Grade 11 chemistry unit 4 Chemical kinetics half life
Chemical kinetics half life Kinetics ap chemistry
Kinetics ap chemistry Chemical kinetics definition
Chemical kinetics definition Chemical kinetics experiment
Chemical kinetics experiment Applications of chemical kinetics
Applications of chemical kinetics Define steady state approximation
Define steady state approximation Sample atkins diet phase 1
Sample atkins diet phase 1 Brenda judd and sandra colley
Brenda judd and sandra colley Nichole atkins
Nichole atkins Atkins fizikai kémia
Atkins fizikai kémia Uncc library databases
Uncc library databases What is a reflection in writing
What is a reflection in writing Paula atkins
Paula atkins Types of ergonomics
Types of ergonomics Atkins or fadkins
Atkins or fadkins Atkins lifestyle
Atkins lifestyle Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Kinetics and equilibrium
Kinetics and equilibrium Prostagalndins
Prostagalndins Planar kinematics of a rigid body
Planar kinematics of a rigid body Antagonistic effect
Antagonistic effect Zero order kinetics
Zero order kinetics Collision theory of kinetics
Collision theory of kinetics Kinetics flotation reagents
Kinetics flotation reagents Kinetics of rigid bodies
Kinetics of rigid bodies Motion of rigid body in three dimensions
Motion of rigid body in three dimensions Kinetics of a particle: force and acceleration
Kinetics of a particle: force and acceleration Kinetics of crystal violet fading
Kinetics of crystal violet fading Octet biosensors
Octet biosensors General principles of catalysis
General principles of catalysis Lineweaver-burk equation
Lineweaver-burk equation Kinetics of particles newton's second law
Kinetics of particles newton's second law Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Dynafit kinetics
Dynafit kinetics Enzyme kinetics
Enzyme kinetics Myeloblast
Myeloblast Planar kinematics of a rigid body
Planar kinematics of a rigid body Data kinetics ltd
Data kinetics ltd Kcat
Kcat Planar kinetics of a rigid body work and energy
Planar kinetics of a rigid body work and energy Kinetics reaction
Kinetics reaction Rate of growth
Rate of growth Kinetics is the branch of
Kinetics is the branch of Fermenter
Fermenter Vampnets for deep learning of molecular kinetics
Vampnets for deep learning of molecular kinetics Pixl knowit gcse chemistry quantitative
Pixl knowit gcse chemistry quantitative Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 A chemist shorthand way of representing chemical reaction.
A chemist shorthand way of representing chemical reaction. Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities