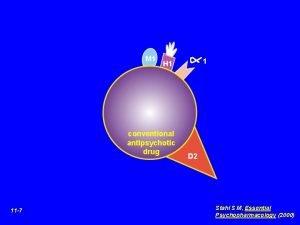
Antipsychotic Chlorpromazine Fluphenazine Mesoridazine Methotrimeprazine Perphenazine Pipotiazine Prochlorperazine














![(Methotrimeprazine) (R)-10 -[ 3 -(dimethylamino)-2 -methylpropyl]-2 methoxyphenothiazine (Methotrimeprazine) (R)-10 -[ 3 -(dimethylamino)-2 -methylpropyl]-2 methoxyphenothiazine](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-20.jpg)
![(RS)-10 -[2 -(1 - methyl-2 piperidyl)ethyl]-2(methylthio)phenothiazine 10 -[3 -(4 - methyl-1 piperazinyl)propyl]-2 trifluoromethylphenothiazine (RS)-10 -[2 -(1 - methyl-2 piperidyl)ethyl]-2(methylthio)phenothiazine 10 -[3 -(4 - methyl-1 piperazinyl)propyl]-2 trifluoromethylphenothiazine](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-22.jpg)

![Flupentixol DEFINITION 2 -[4 -[3 -[(EZ)-2 -(trifluoromethyl)-9 Hthioxanthen-9 -ylidene]propyl]piperazin -1 -yl]ethanol dihydrochloride. Content - Flupentixol DEFINITION 2 -[4 -[3 -[(EZ)-2 -(trifluoromethyl)-9 Hthioxanthen-9 -ylidene]propyl]piperazin -1 -yl]ethanol dihydrochloride. Content -](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-26.jpg)


- Slides: 32



• Antipsychotic — Chlorpromazine; Fluphenazine; Mesoridazine; Methotrimeprazine; Perphenazine; Pipotiazine; Prochlorperazine; Promazine; Thioproperazine; Thioridazine; Trifluoperazine; Triflupromazine; • Antipsychotic adjunct — Pericyazine; • Anesthetic adjunct — Chlorpromazine; Methotrimeprazine, intravenous; • Antidyskinetic, Huntington's chorea — Chlorpromazine; Thioridazine; • Antiemetic — Chlorpromazine; Methotrimeprazine; Perphenazine; Prochlorperazine; Trifluoperazine; Triflupromazine; • Antineuralgia adjunct — Fluphenazine; • Sedative — Chlorpromazine; Methotrimeprazine; Thioridazine; • Analgesic — Methotrimeprazine;







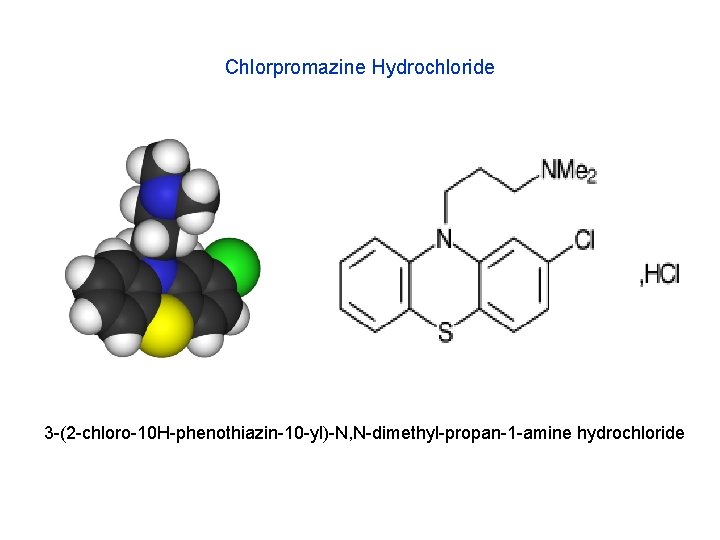
Chlorpromazine Hydrochloride 3 -(2 -chloro-10 H-phenothiazin-10 -yl)-N, N-dimethyl-propan-1 -amine hydrochloride


IDENTIFICATION OF PHENOTHIAZINES (Ph. Eur. method 2. 3. 3) Carry out the method for thin-layer chromatography protected from light using kieselguhr G as the coating substance. Impregnate the dry plate by placing it in a tank containing a shallow layer of a solution containing 10% v/v of 2 -phenoxyethanol and 5. 0% w/v of polyethylene glycol 300 in acetone so that the plate dips about 5 mm beneath the surface of the liquid and allowing the impregnating solvent to ascend at least 17 cm above the line of application. Remove the plate from the tank and use it immediately. Carry out the chromatography in the same direction as the impregnation. For the mobile phase shake a mixture of 100 volumes of petroleum spirit (boiling range, 50° to 70°) and 2 volumes of diethylamine with 6 to 8 volumes of 2 -phenoxyethanol until a persistent cloudiness is obtained, decant and use the supernatant layer even if cloudy. Apply separately to the plate 2 µl of each of two solutions in chloroform containing (1) 0. 2% w/v of the substance being examined and (2) 0. 2% w/v of the corresponding European Pharmacopoeia Chemical Reference Substance. After removal of the plate, examine under ultraviolet light (365 nm) and observe the fluorescence produced after a few minutes. The spot in the chromatogram obtained with solution (1) is similar in position, colour, fluorescence and size to that in the chromatogram obtained with solution (2). Spray the plate with ethanolic sulphuric acid ( 10%) and observe the colour produced. The colour of the spot in the chromatogram obtained with solution (1) is the same as that in the chromatogram obtained with solution (2) and has a similar stability over a period of at least 20 minutes after spraying.

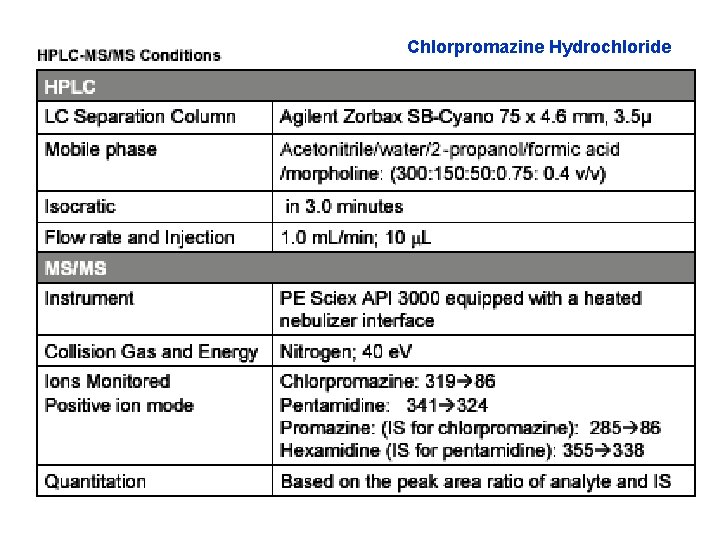
Chlorpromazine Hydrochloride

Abstract: An automated on-line method for simultaneous analysis of five phenothiazine drugs by high-performance liquid chromatography (HPLC)/sonic spray ionization mass spectrometry (SSI-MS) has been established, using backflush column switching. A 400 -μl portion of serum sample diluted 81 -fold with distilled water was subjected to the on-line system. In the system, an Oasis HLB cartridge was used as the precolumn for extraction; large molecules such as proteins in serum were discarded by use of distilled water containing 0. 1% formic acid as a mobile phase. After switching a valve, the analytes trapped in the precolumn were eluted in the backflush mode and separated by a Chromolith Performance RP 18 e column, which is composed of C 18 -bonded monolithic silica. The column effluents were then introduced into the SSI-MS. The present method provided successful separation and determination of six phenothiazines including an internal standard. Satisfactory linearities, reproducibility, and sensitivity were obtained at concentration levels that matched the toxic levels of phenothiazines. All drug peaks appeared within 18 min, and the system could be reequilibrated in only about 8 min for the next run. Because of the simplicity and rapidness of the method, it is likely to be useful in the fields of emergency medicine and forensic toxicology.

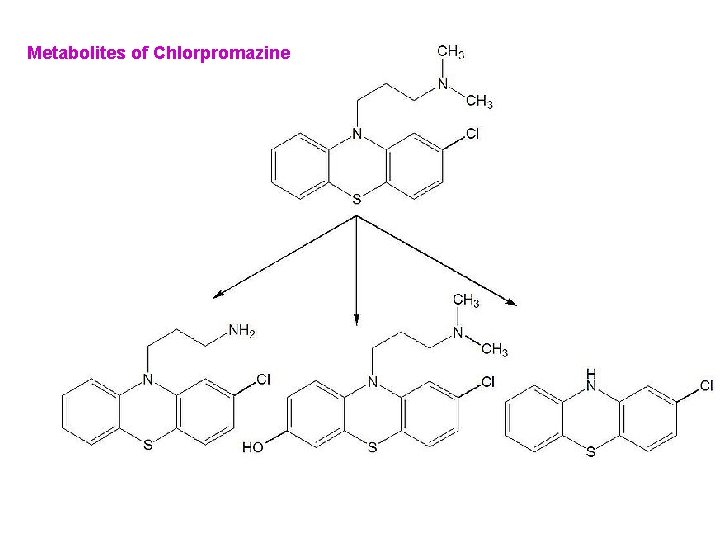
Metabolites of Chlorpromazine


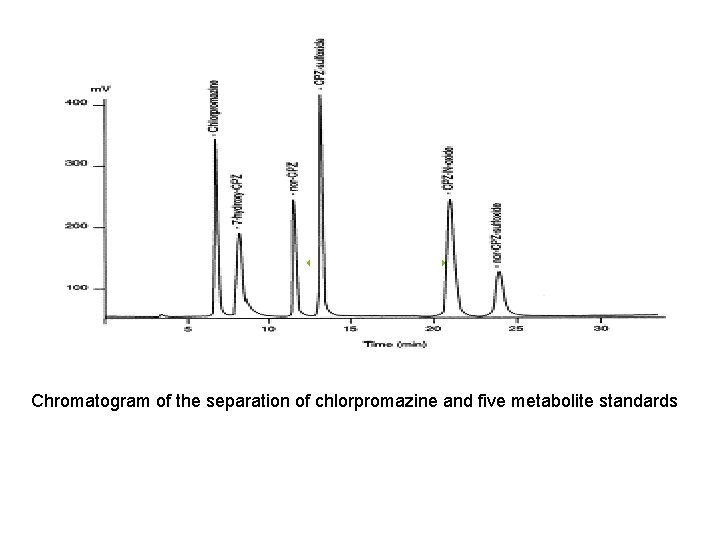
Chromatogram of the separation of chlorpromazine and five metabolite standards


![Methotrimeprazine R10 3 dimethylamino2 methylpropyl2 methoxyphenothiazine (Methotrimeprazine) (R)-10 -[ 3 -(dimethylamino)-2 -methylpropyl]-2 methoxyphenothiazine](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-20.jpg)
(Methotrimeprazine) (R)-10 -[ 3 -(dimethylamino)-2 -methylpropyl]-2 methoxyphenothiazine

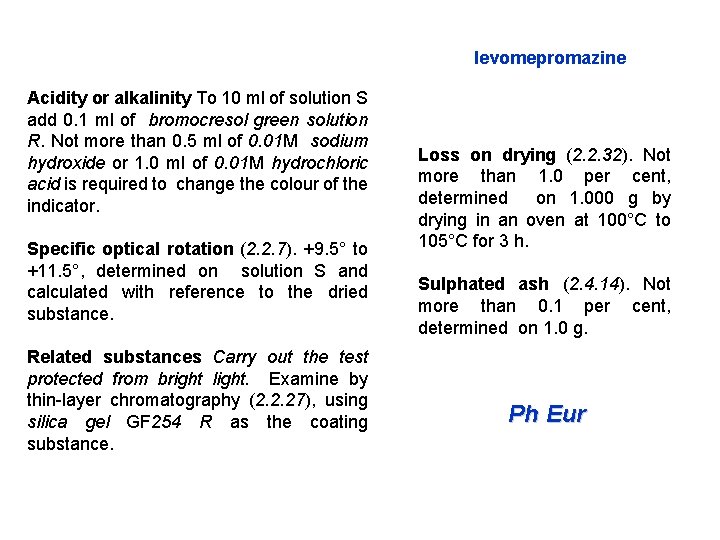
levomepromazine Acidity or alkalinity To 10 ml of solution S add 0. 1 ml of bromocresol green solution R. Not more than 0. 5 ml of 0. 01 M sodium hydroxide or 1. 0 ml of 0. 01 M hydrochloric acid is required to change the colour of the indicator. Specific optical rotation (2. 2. 7). +9. 5° to +11. 5°, determined on solution S and calculated with reference to the dried substance. Related substances Carry out the test protected from bright light. Examine by thin-layer chromatography (2. 2. 27), using silica gel GF 254 R as the coating substance. Loss on drying (2. 2. 32). Not more than 1. 0 per cent, determined on 1. 000 g by drying in an oven at 100°C to 105°C for 3 h. Sulphated ash (2. 4. 14). Not more than 0. 1 per cent, determined on 1. 0 g. Ph Eur
![RS10 2 1 methyl2 piperidylethyl2methylthiophenothiazine 10 3 4 methyl1 piperazinylpropyl2 trifluoromethylphenothiazine (RS)-10 -[2 -(1 - methyl-2 piperidyl)ethyl]-2(methylthio)phenothiazine 10 -[3 -(4 - methyl-1 piperazinyl)propyl]-2 trifluoromethylphenothiazine](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-22.jpg)
(RS)-10 -[2 -(1 - methyl-2 piperidyl)ethyl]-2(methylthio)phenothiazine 10 -[3 -(4 - methyl-1 piperazinyl)propyl]-2 trifluoromethylphenothiazine

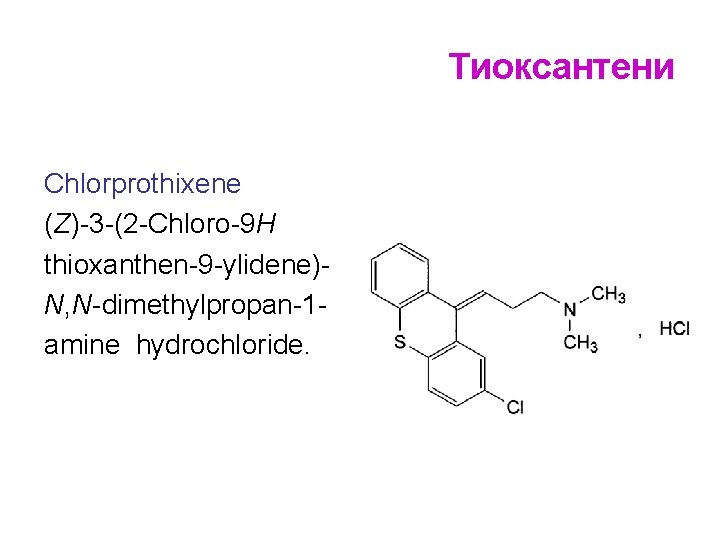
Тиоксантени Chlorprothixene (Z)-3 -(2 -Chloro-9 H thioxanthen-9 -ylidene)N, N-dimethylpropan-1 amine hydrochloride.


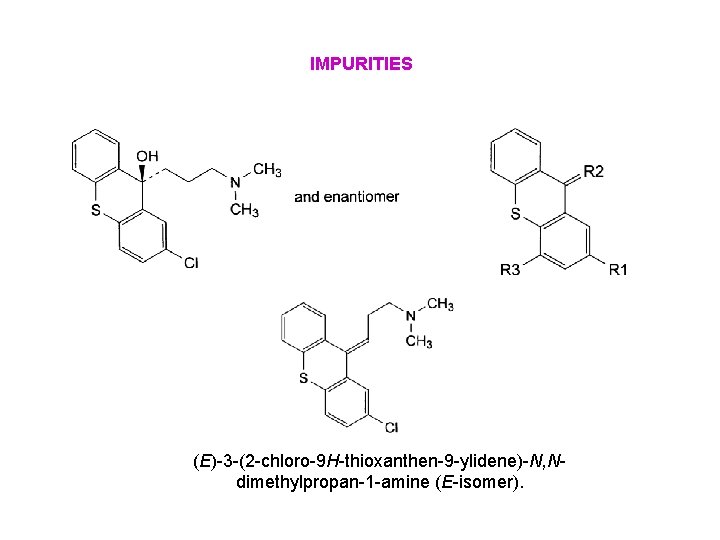
IMPURITIES (E)-3 -(2 -chloro-9 H-thioxanthen-9 -ylidene)-N, Ndimethylpropan-1 -amine (E-isomer).
![Flupentixol DEFINITION 2 4 3 EZ2 trifluoromethyl9 Hthioxanthen9 ylidenepropylpiperazin 1 ylethanol dihydrochloride Content Flupentixol DEFINITION 2 -[4 -[3 -[(EZ)-2 -(trifluoromethyl)-9 Hthioxanthen-9 -ylidene]propyl]piperazin -1 -yl]ethanol dihydrochloride. Content -](https://slidetodoc.com/presentation_image_h2/7b3bef001bdab86a71e03ff136ca44b1/image-26.jpg)
Flupentixol DEFINITION 2 -[4 -[3 -[(EZ)-2 -(trifluoromethyl)-9 Hthioxanthen-9 -ylidene]propyl]piperazin -1 -yl]ethanol dihydrochloride. Content - flupentixol dihydrochloride: 98. 0 per cent to 101. 5 per cent (dried substance), - Z-isomer: 42. 0 per cent to 52. 0 per cent. 2 -(trifluoromethyl)-9 H-thioxanthen-9 -one.

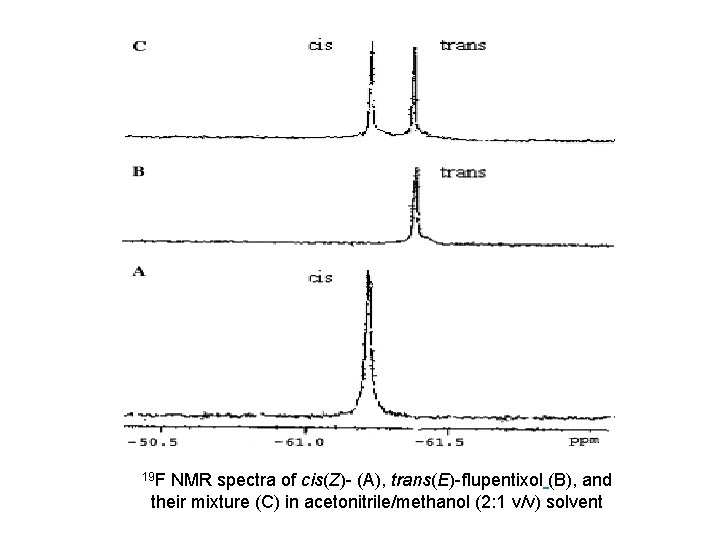
The structure of flupentixol is based on the thioxanthene ring to which the side chain is linked by a C=C double bond. Because of the double bond linking of the side chain to the thioxanthene ring structure, flupentixol exists as two geometric isomers, namely cis (Z) and trans (E) isomers. It is interesting to note that, in pharmacological studies, the neuroleptic activity is associated with the cis (Z) isomer, while the trans (E) isomer is practically inactive. The oral form of flupentixol, administered as tablets or drops, is an equimolar mixture of the active cis (Z) and the inactive trans (E) isomers, while the parenteral preparation for intramuscular injection is the decanoic acid ester of the isolated cis (Z) isomer.


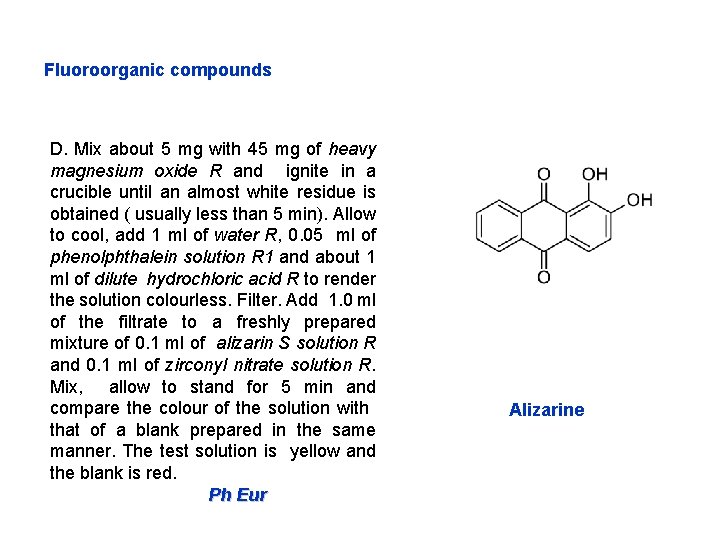
Fluoroorganic compounds D. Mix about 5 mg with 45 mg of heavy magnesium oxide R and ignite in a crucible until an almost white residue is obtained ( usually less than 5 min). Allow to cool, add 1 ml of water R, 0. 05 ml of phenolphthalein solution R 1 and about 1 ml of dilute hydrochloric acid R to render the solution colourless. Filter. Add 1. 0 ml of the filtrate to a freshly prepared mixture of 0. 1 ml of alizarin S solution R and 0. 1 ml of zirconyl nitrate solution R. Mix, allow to stand for 5 min and compare the colour of the solution with that of a blank prepared in the same manner. The test solution is yellow and the blank is red. Ph Eur Alizarine

Title: METHOD AND APPARATUS FOR DETERMINATION OF CARBON-HALOGEN COMPOUNDS AND APPLICATIONS THEREOF Abstract: A method and apparatus for determination of fluoroorganic compounds in liquid gaseous, or crystalline or amorphous solids is based on the detection of carbon-halogen bonds by laser Raman spectroscopy. The method and apparatus provide a general method for detecting and determination of haloorganic compounds. The method and apparatus are applicable in the pharmaceutical industry, in the fluorinated drug research and manufacturing in the medical and clinical studies of the effects of fluoroorganic compounds, in the environmental and agricultural studies and screening, in the analysis of water, soils and air contaminated with fluoroorganic compounds.

As a basis for quantitative determination of fluorinate species, flourine-19 nuclear magnetic resonance (19 F NMR) offers several potential advantages compared to chromatography. 19 F NMR spectroscopy has a large spectral window associated with it. Typically organofluorine nuclei lie within a window of 300 ppm, resulting in a small probability of peak overlap between molecules. Moreover, the spin 1/2 19 F isotope is 100% abundant and possesses a sensitivity which is 81% of a 1 H nucleus. The technique can be used for spectral identification of an analyte and for quantification, when an internal standard of known concentration is included

19 F NMR spectra of cis(Z)- (A), trans(E)- flupentixol (B), and their mixture (C) in acetonitrile/methanol (2: 1 v/v) solvent