Anti retroviral Pharmacology Antiretroviral Classes NRTIs Nucleoside OR






- Slides: 34

Anti retroviral Pharmacology

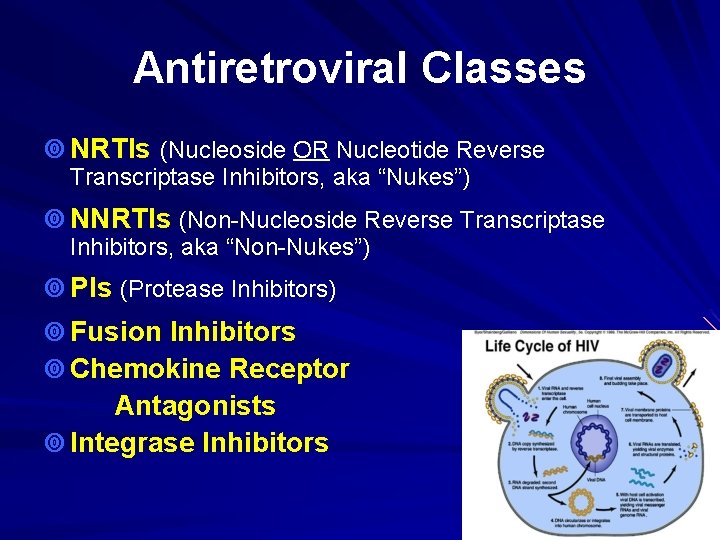
Antiretroviral Classes ¥ NRTIs (Nucleoside OR Nucleotide Reverse Transcriptase Inhibitors, aka “Nukes”) ¥ NNRTIs (Non-Nucleoside Reverse Transcriptase Inhibitors, aka “Non-Nukes”) ¥ PIs (Protease Inhibitors) ¥ Fusion Inhibitors ¥ Chemokine Receptor Antagonists ¥ Integrase Inhibitors

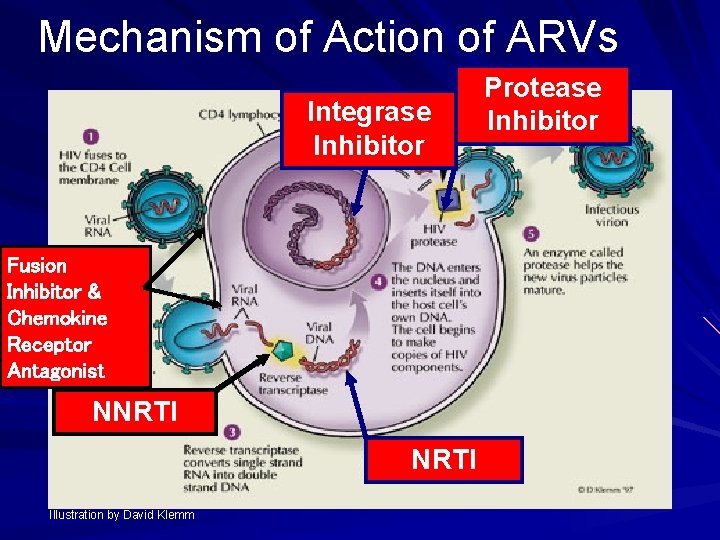
Mechanism of Action of ARVs Integrase Inhibitor Fusion Inhibitor & Chemokine Receptor Antagonist NNRTI Illustration by David Klemm Protease Inhibitor

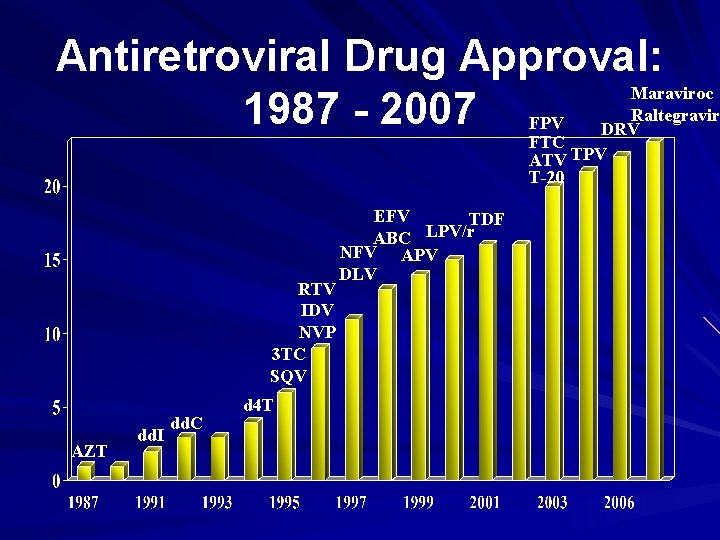
Antiretroviral Drug Approval: Maraviroc 1987 - 2007 FPV DRVRaltegravir FTC ATV TPV T-20 RTV IDV NVP 3 TC SQV AZT dd. I dd. C d 4 T EFV TDF LPV/r ABC NFV APV DLV

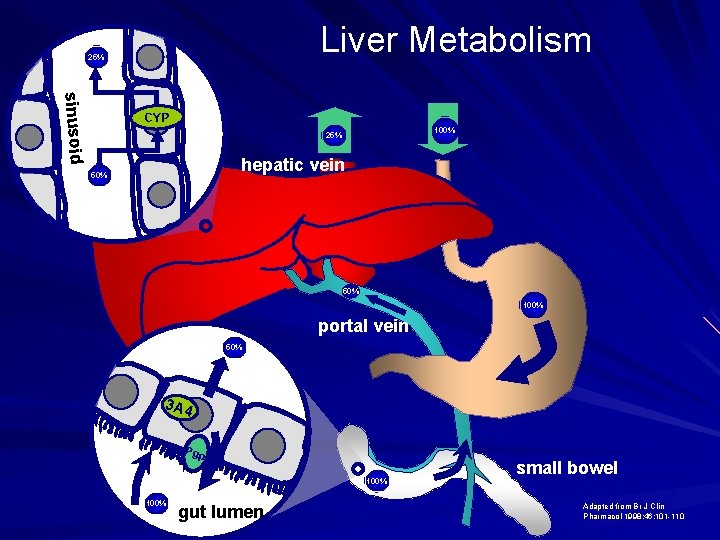
Liver Metabolism 25% sinusoid CYP 100% 25% hepatic vein 50% 100% portal vein 50% 3 A 4 Pgp 100% gut lumen small bowel Adapted from Br J Clin Pharmacol 1998: 46: 101 -110

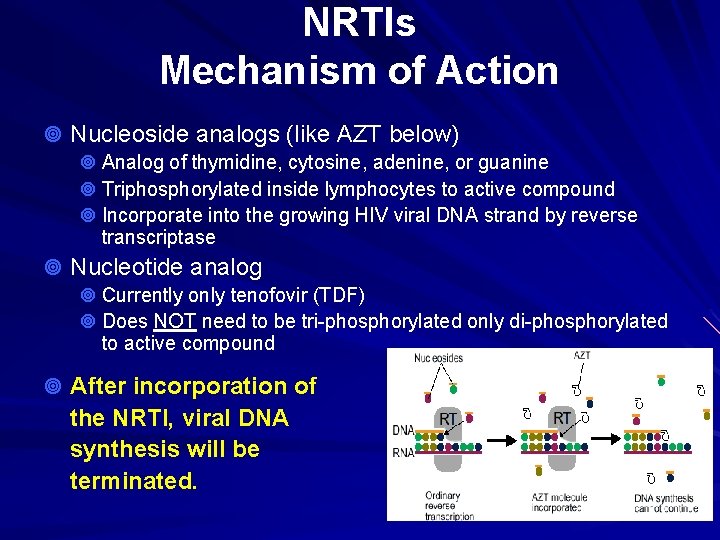
NRTIs Mechanism of Action ¥ Nucleoside analogs (like AZT below) ¥ Analog of thymidine, cytosine, adenine, or guanine ¥ Triphosphorylated inside lymphocytes to active compound ¥ Incorporate into the growing HIV viral DNA strand by reverse transcriptase ¥ Nucleotide analog ¥ Currently only tenofovir (TDF) ¥ Does NOT need to be tri-phosphorylated only di-phosphorylated to active compound ¥ After incorporation of the NRTI, viral DNA synthesis will be terminated.

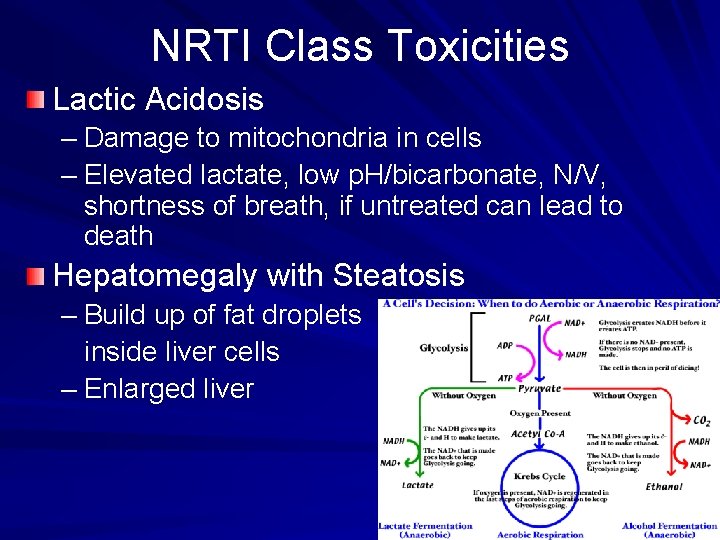
NRTI Class Toxicities Lactic Acidosis – Damage to mitochondria in cells – Elevated lactate, low p. H/bicarbonate, N/V, shortness of breath, if untreated can lead to death Hepatomegaly with Steatosis – Build up of fat droplets inside liver cells – Enlarged liver

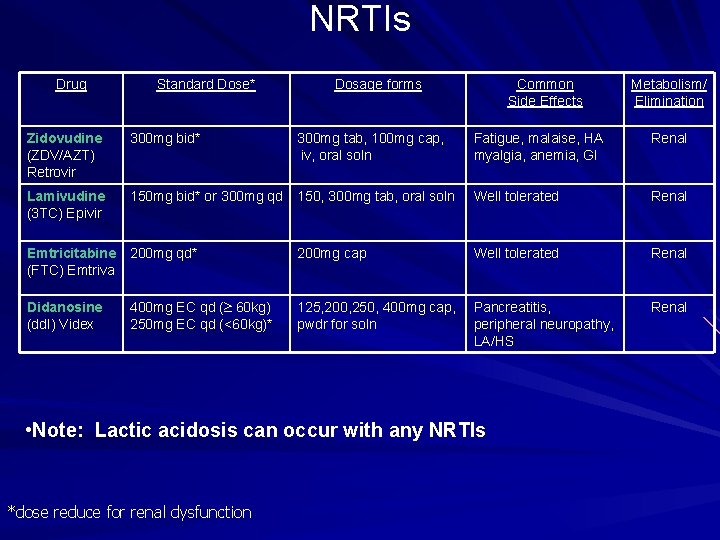
NRTIs Drug Standard Dose* Dosage forms Common Side Effects Metabolism/ Elimination Zidovudine (ZDV/AZT) Retrovir 300 mg bid* 300 mg tab, 100 mg cap, iv, oral soln Fatigue, malaise, HA myalgia, anemia, GI Renal Lamivudine (3 TC) Epivir 150 mg bid* or 300 mg qd 150, 300 mg tab, oral soln Well tolerated Renal Emtricitabine (FTC) Emtriva 200 mg qd* 200 mg cap Well tolerated Renal Didanosine (dd. I) Videx 400 mg EC qd ( 60 kg) 250 mg EC qd (<60 kg)* 125, 200, 250, 400 mg cap, pwdr for soln Pancreatitis, peripheral neuropathy, LA/HS Renal • Note: Lactic acidosis can occur with any NRTIs *dose reduce for renal dysfunction

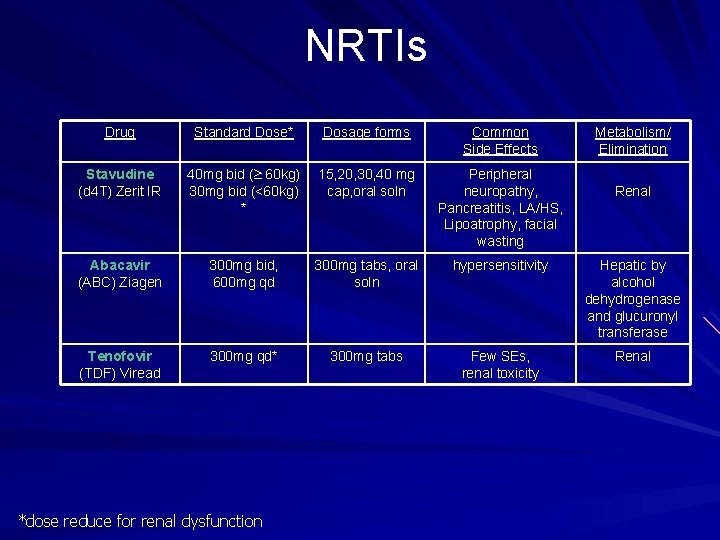
NRTIs Drug Standard Dose* Dosage forms Common Side Effects Stavudine (d 4 T) Zerit IR 40 mg bid ( 60 kg) 30 mg bid (<60 kg) * 15, 20, 30, 40 mg cap, oral soln Peripheral neuropathy, Pancreatitis, LA/HS, Lipoatrophy, facial wasting Abacavir (ABC) Ziagen 300 mg bid, 600 mg qd 300 mg tabs, oral soln hypersensitivity Hepatic by alcohol dehydrogenase and glucuronyl transferase Tenofovir (TDF) Viread 300 mg qd* 300 mg tabs Few SEs, renal toxicity Renal *dose reduce for renal dysfunction Metabolism/ Elimination Renal

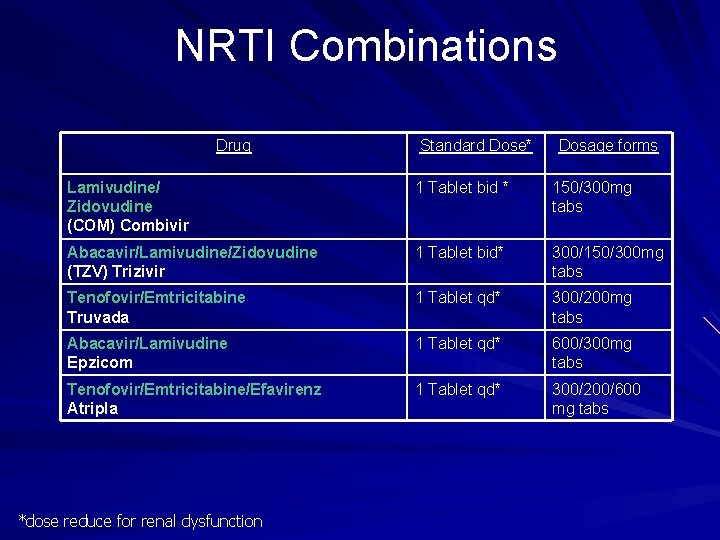
NRTI Combinations Drug Standard Dose* Dosage forms Lamivudine/ Zidovudine (COM) Combivir 1 Tablet bid * 150/300 mg tabs Abacavir/Lamivudine/Zidovudine (TZV) Trizivir 1 Tablet bid* 300/150/300 mg tabs Tenofovir/Emtricitabine Truvada 1 Tablet qd* 300/200 mg tabs Abacavir/Lamivudine Epzicom 1 Tablet qd* 600/300 mg tabs Tenofovir/Emtricitabine/Efavirenz Atripla 1 Tablet qd* 300/200/600 mg tabs *dose reduce for renal dysfunction

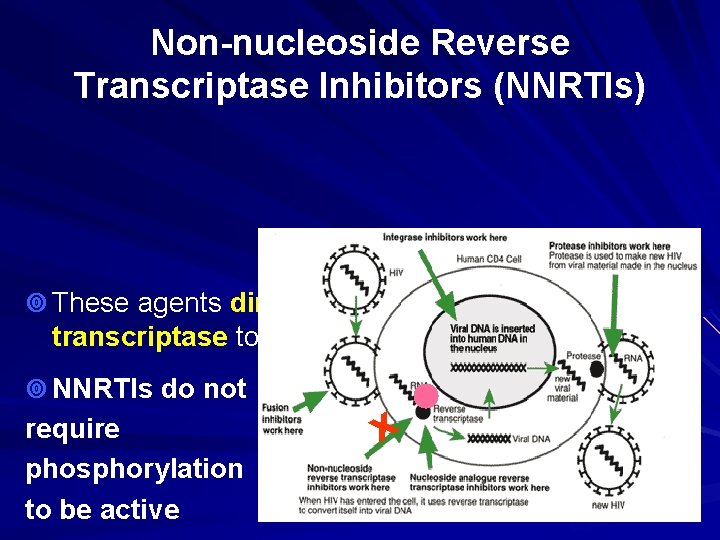
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs) ¥ These agents directly bind to reverse RT transcriptase to inhibit transcription ¥ NNRTIs do not require phosphorylation to be active X

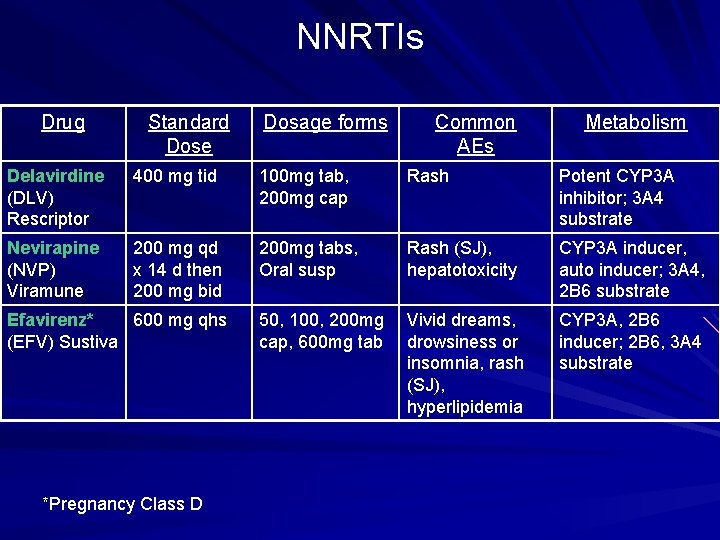
NNRTIs Drug Standard Dose Dosage forms Common AEs Metabolism Delavirdine (DLV) Rescriptor 400 mg tid 100 mg tab, 200 mg cap Rash Potent CYP 3 A inhibitor; 3 A 4 substrate Nevirapine (NVP) Viramune 200 mg qd x 14 d then 200 mg bid 200 mg tabs, Oral susp Rash (SJ), hepatotoxicity CYP 3 A inducer, auto inducer; 3 A 4, 2 B 6 substrate 50, 100, 200 mg cap, 600 mg tab Vivid dreams, drowsiness or insomnia, rash (SJ), hyperlipidemia CYP 3 A, 2 B 6 inducer; 2 B 6, 3 A 4 substrate Efavirenz* 600 mg qhs (EFV) Sustiva *Pregnancy Class D

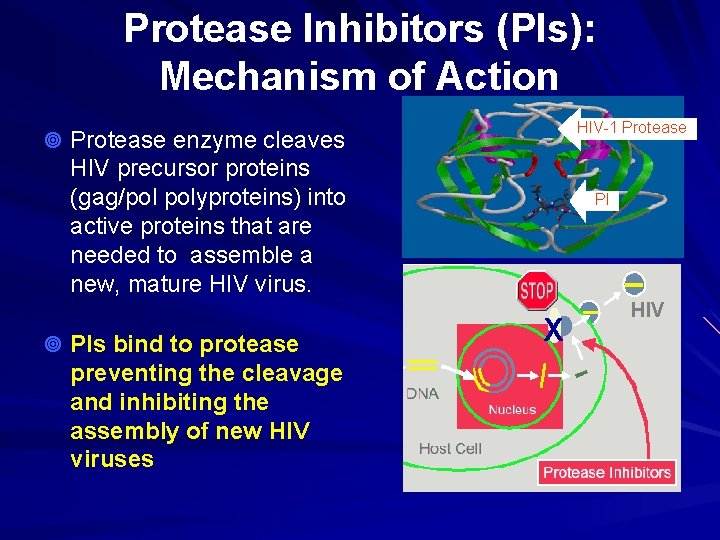
Protease Inhibitors (PIs): Mechanism of Action HIV-1 Protease ¥ Protease enzyme cleaves HIV precursor proteins (gag/pol polyproteins) into active proteins that are needed to assemble a new, mature HIV virus. ¥ PIs bind to protease preventing the cleavage and inhibiting the assembly of new HIV viruses PI X HIV

Lipids, Insulin Resistance (Lypodystrophy) Hypercolesterolemia – Usually hypertriglyceridemia, can have increased LDL and decreased HDL – Treat with Fibric acid derivatives and certain HMGCo. A reductase inhibitors Insulin Resistance – Treat with diet/exercise, metformin, TZDs, insulin, sulfonylureas

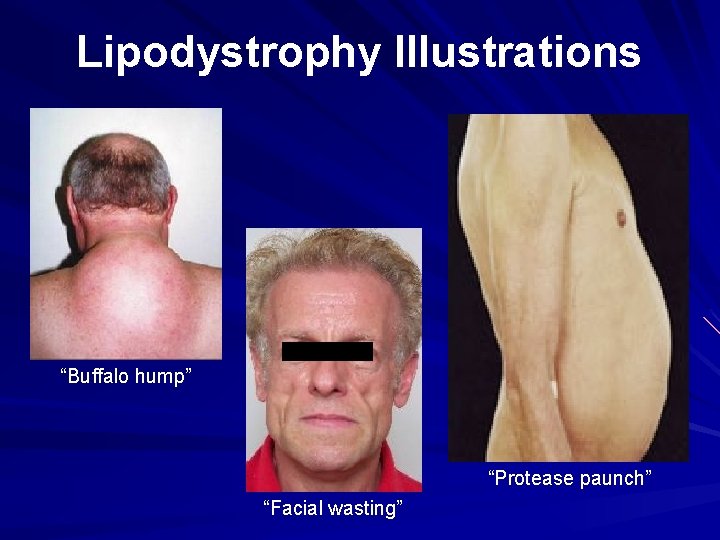
Lipodystrophy Illustrations “Buffalo hump” “Protease paunch” “Facial wasting”

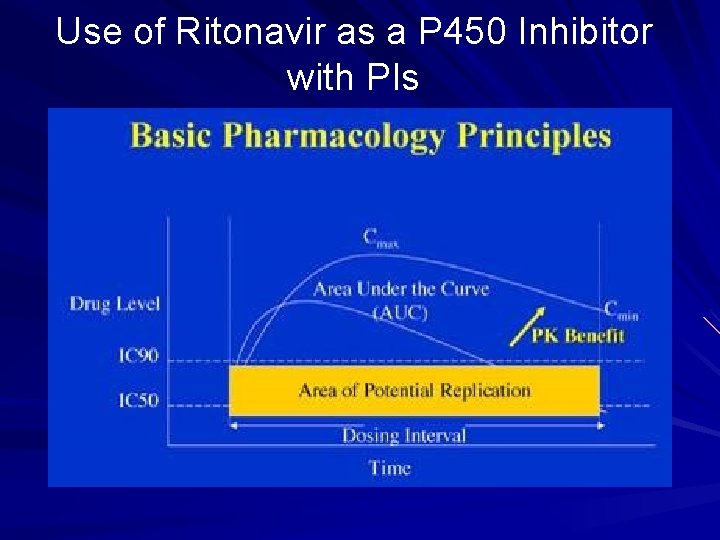
Use of Ritonavir as a P 450 Inhibitor with PIs

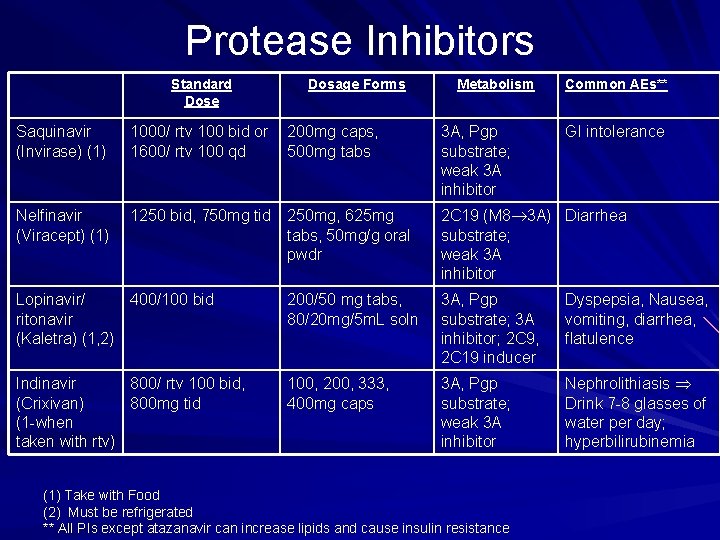
Protease Inhibitors Standard Dose Dosage Forms Metabolism Common AEs** Saquinavir (Invirase) (1) 1000/ rtv 100 bid or 1600/ rtv 100 qd 200 mg caps, 500 mg tabs 3 A, Pgp substrate; weak 3 A inhibitor Nelfinavir (Viracept) (1) 1250 bid, 750 mg tid 250 mg, 625 mg tabs, 50 mg/g oral pwdr 2 C 19 (M 8 3 A) Diarrhea substrate; weak 3 A inhibitor Lopinavir/ 400/100 bid ritonavir (Kaletra) (1, 2) 200/50 mg tabs, 80/20 mg/5 m. L soln 3 A, Pgp substrate; 3 A inhibitor; 2 C 9, 2 C 19 inducer Dyspepsia, Nausea, vomiting, diarrhea, flatulence Indinavir 800/ rtv 100 bid, (Crixivan) 800 mg tid (1 -when taken with rtv) 100, 200, 333, 400 mg caps 3 A, Pgp substrate; weak 3 A inhibitor Nephrolithiasis Drink 7 -8 glasses of water per day; hyperbilirubinemia (1) Take with Food (2) Must be refrigerated ** All PIs except atazanavir can increase lipids and cause insulin resistance GI intolerance

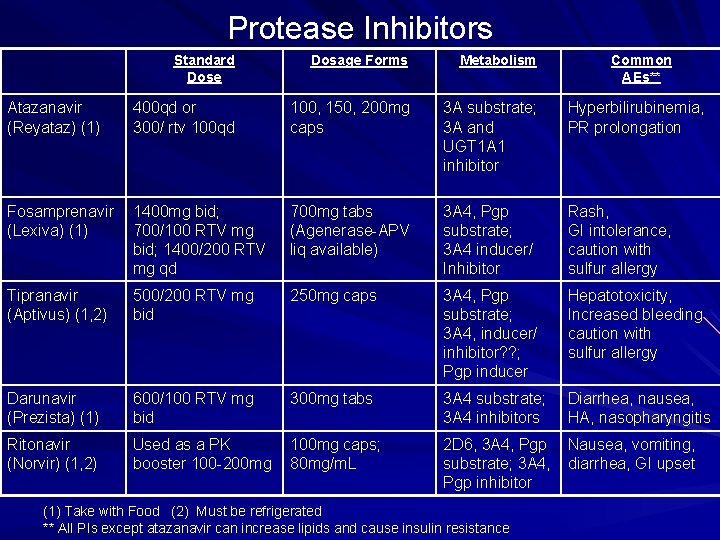
Protease Inhibitors Standard Dose Dosage Forms Metabolism Atazanavir (Reyataz) (1) 400 qd or 300/ rtv 100 qd 100, 150, 200 mg caps 3 A substrate; 3 A and UGT 1 A 1 inhibitor Hyperbilirubinemia, PR prolongation Fosamprenavir (Lexiva) (1) 1400 mg bid; 700/100 RTV mg bid; 1400/200 RTV mg qd 700 mg tabs (Agenerase-APV liq available) 3 A 4, Pgp substrate; 3 A 4 inducer/ Inhibitor Rash, GI intolerance, caution with sulfur allergy Tipranavir (Aptivus) (1, 2) 500/200 RTV mg bid 250 mg caps 3 A 4, Pgp substrate; 3 A 4, inducer/ inhibitor? ? ; Pgp inducer Hepatotoxicity, Increased bleeding caution with sulfur allergy Darunavir (Prezista) (1) 600/100 RTV mg bid 300 mg tabs 3 A 4 substrate; 3 A 4 inhibitors Diarrhea, nausea, HA, nasopharyngitis Ritonavir (Norvir) (1, 2) Used as a PK booster 100 -200 mg 100 mg caps; 80 mg/m. L 2 D 6, 3 A 4, Pgp substrate; 3 A 4, Pgp inhibitor Nausea, vomiting, diarrhea, GI upset (1) Take with Food (2) Must be refrigerated ** All PIs except atazanavir can increase lipids and cause insulin resistance Common AEs**

Dose adjustments to consider Renally-eliminated NRTIs (except Abacavir) Adjust for Cr. Cl <50 ml/min or dialysis Didanosine Emtricitabine Lamivudine Stavudine Tenofovir Zidovudine Reference: Drug product info and DHHS guidelines (see tables) Hepatic Metabolism NNRTIs PIs Adjust for certain inducers, substrates, or inhibitors of P 450 system Adjust for insufficiency Indinavir Fosamprenavir Atazanavir Avoid Amprenavir oral soln Foasmprenavir (+/- ritonavir) Tipranavir

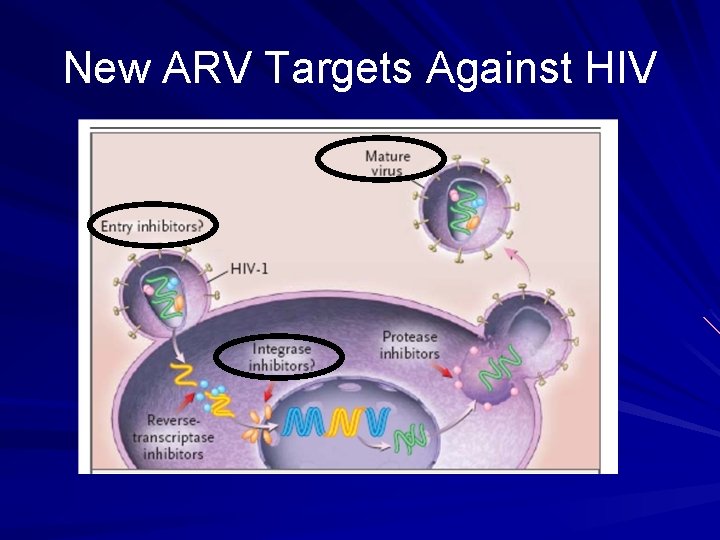
New ARV Targets Against HIV

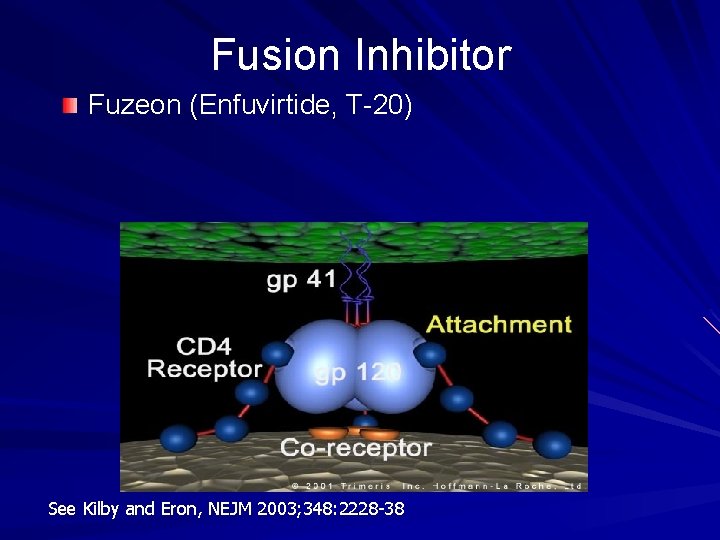
Fusion Inhibitor Fuzeon (Enfuvirtide, T-20) See Kilby and Eron, NEJM 2003; 348: 2228 -38

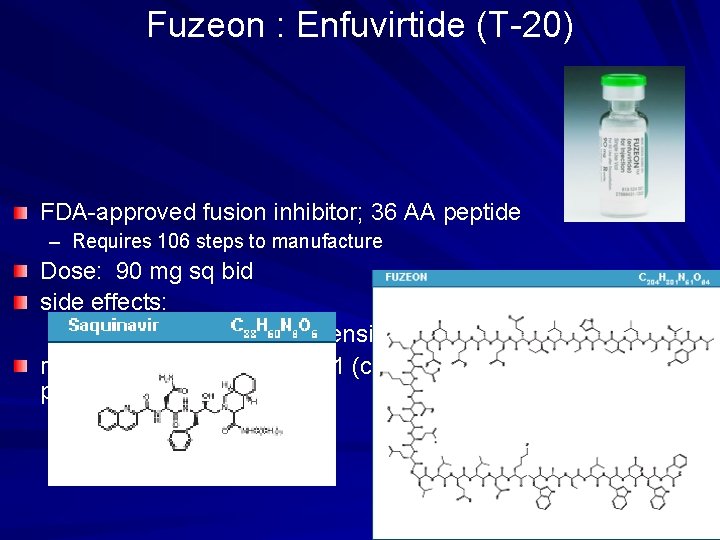
Fuzeon : Enfuvirtide (T-20) FDA-approved fusion inhibitor; 36 AA peptide – Requires 106 steps to manufacture Dose: 90 mg sq bid side effects: – injection site rxn, hypersensitivity (rare) resistance: changes in gp 41 (cell surface protein)

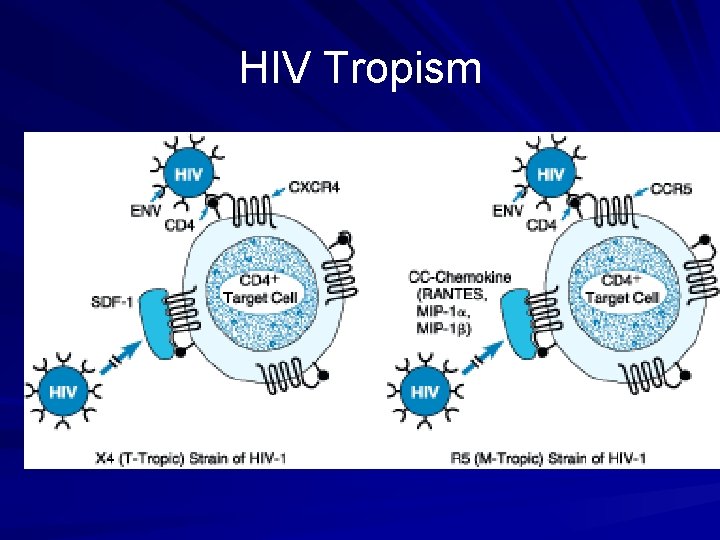
HIV Tropism

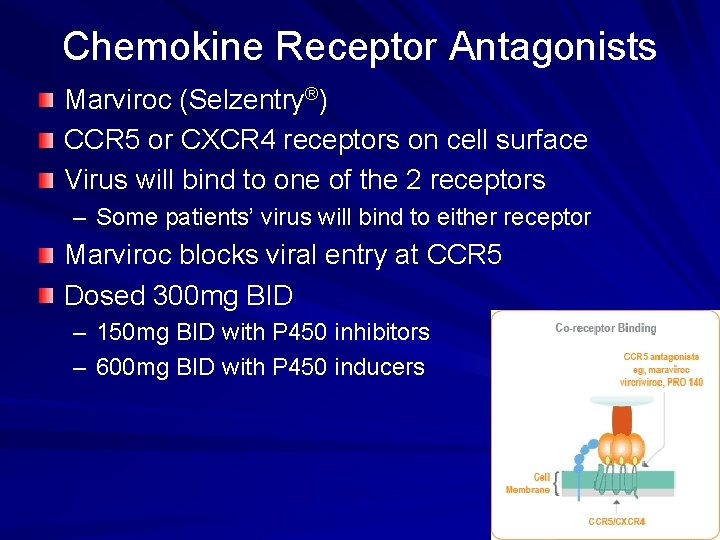
Chemokine Receptor Antagonists Marviroc (Selzentry®) CCR 5 or CXCR 4 receptors on cell surface Virus will bind to one of the 2 receptors – Some patients’ virus will bind to either receptor Marviroc blocks viral entry at CCR 5 Dosed 300 mg BID – 150 mg BID with P 450 inhibitors – 600 mg BID with P 450 inducers

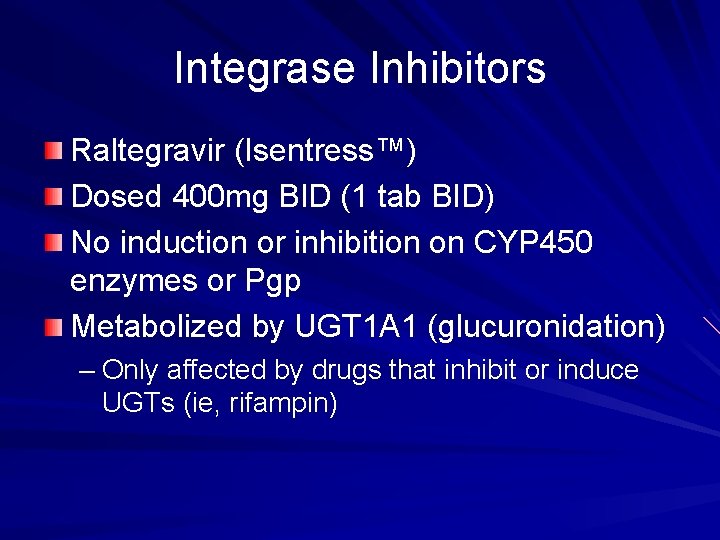
Integrase Inhibitors Raltegravir (Isentress™) Dosed 400 mg BID (1 tab BID) No induction or inhibition on CYP 450 enzymes or Pgp Metabolized by UGT 1 A 1 (glucuronidation) – Only affected by drugs that inhibit or induce UGTs (ie, rifampin)

Drug Interactions

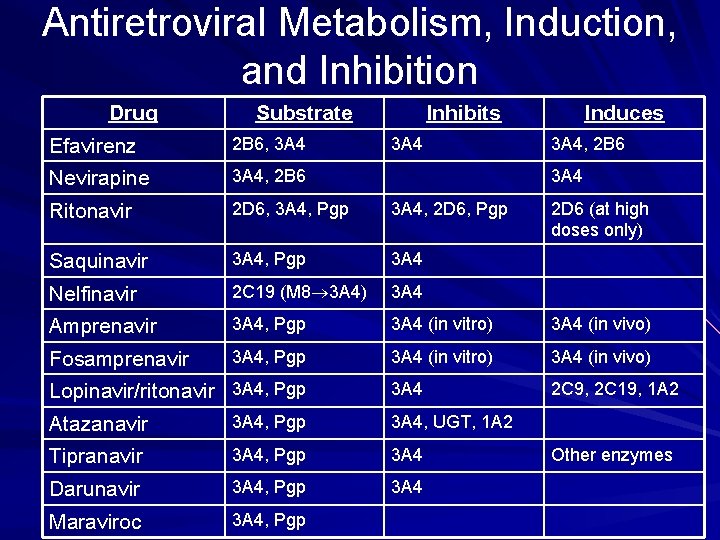
Antiretroviral Metabolism, Induction, and Inhibition Drug Substrate Inhibits Efavirenz 2 B 6, 3 A 4 Nevirapine 3 A 4, 2 B 6 Ritonavir 2 D 6, 3 A 4, Pgp 3 A 4, 2 D 6, Pgp Saquinavir 3 A 4, Pgp 3 A 4 Nelfinavir 2 C 19 (M 8 3 A 4) 3 A 4 Amprenavir 3 A 4, Pgp 3 A 4 (in vitro) 3 A 4 (in vivo) Fosamprenavir 3 A 4, Pgp 3 A 4 (in vitro) 3 A 4 (in vivo) 3 A 4 2 C 9, 2 C 19, 1 A 2 Lopinavir/ritonavir 3 A 4, Pgp 3 A 4 Induces 3 A 4, 2 B 6 3 A 4 Atazanavir 3 A 4, Pgp 3 A 4, UGT, 1 A 2 Tipranavir 3 A 4, Pgp 3 A 4 Darunavir 3 A 4, Pgp 3 A 4 Maraviroc 3 A 4, Pgp 2 D 6 (at high doses only) Other enzymes

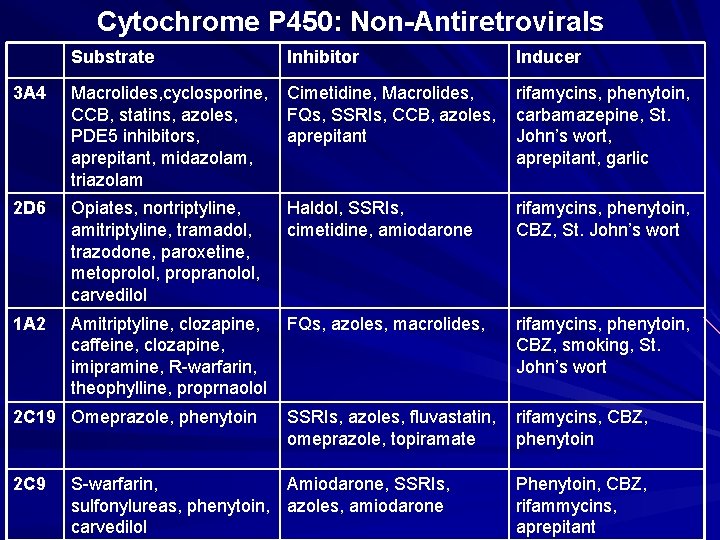
Cytochrome P 450: Non-Antiretrovirals Substrate Inhibitor Inducer 3 A 4 Macrolides, cyclosporine, CCB, statins, azoles, PDE 5 inhibitors, aprepitant, midazolam, triazolam Cimetidine, Macrolides, FQs, SSRIs, CCB, azoles, aprepitant rifamycins, phenytoin, carbamazepine, St. John’s wort, aprepitant, garlic 2 D 6 Opiates, nortriptyline, amitriptyline, tramadol, trazodone, paroxetine, metoprolol, propranolol, carvedilol Haldol, SSRIs, cimetidine, amiodarone rifamycins, phenytoin, CBZ, St. John’s wort 1 A 2 Amitriptyline, clozapine, caffeine, clozapine, imipramine, R-warfarin, theophylline, proprnaolol FQs, azoles, macrolides, rifamycins, phenytoin, CBZ, smoking, St. John’s wort SSRIs, azoles, fluvastatin, omeprazole, topiramate rifamycins, CBZ, phenytoin 2 C 19 Omeprazole, phenytoin 2 C 9 S-warfarin, Amiodarone, SSRIs, sulfonylureas, phenytoin, azoles, amiodarone carvedilol Phenytoin, CBZ, rifammycins, aprepitant

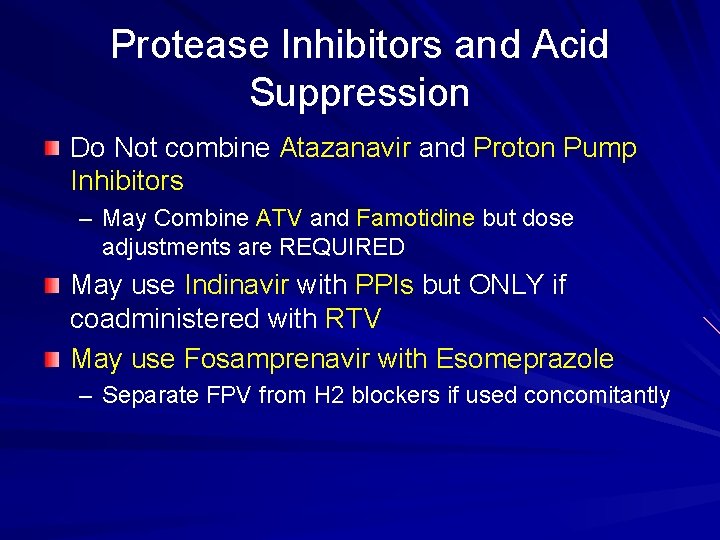
Protease Inhibitors and Acid Suppression Do Not combine Atazanavir and Proton Pump Inhibitors – May Combine ATV and Famotidine but dose adjustments are REQUIRED May use Indinavir with PPIs but ONLY if coadministered with RTV May use Fosamprenavir with Esomeprazole – Separate FPV from H 2 blockers if used concomitantly

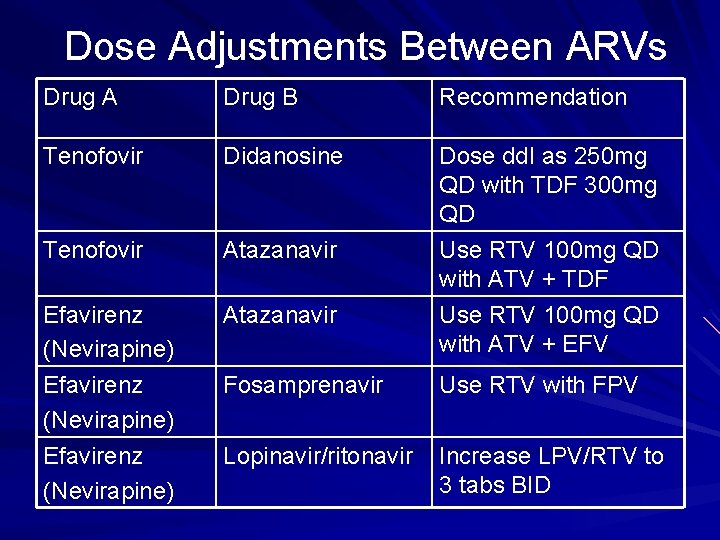
Dose Adjustments Between ARVs Drug A Drug B Recommendation Tenofovir Didanosine Tenofovir Atazanavir Efavirenz (Nevirapine) Atazanavir Dose dd. I as 250 mg QD with TDF 300 mg QD Use RTV 100 mg QD with ATV + TDF Use RTV 100 mg QD with ATV + EFV Fosamprenavir Use RTV with FPV Lopinavir/ritonavir Increase LPV/RTV to 3 tabs BID

Important Drug Interactions Do NOT use Simvastatin, Lovastatin, Antiarrthymics, Midazolam, Triazolam, Ergot derivatives, Rifamin, St. Johns Wort, or Garlic with most PIs or DLV Do NOT combine Rifampin with PIs – LPV/RTV may be dose increased and combined with Rifampin – Conflicting data with EFV and NVP Use other P 450 inducers with CAUTION when combining with PIs and NNRTIs Do NOT use Fluticasone or Alfuzosin with Ritonavir Caution with Azoles, Clarithromycin, Oral Contraceptives, Phenytoin, Carbamazepine, Phenobarbital, Methadone, PDE 5 inhibitors, Atorvastatin, Beta blockers, when combined with PIs Avoid Herbal Products with Known or Suspected Interactions When combining Protease Inhibitors, Often Dose Adjustments are Necessary

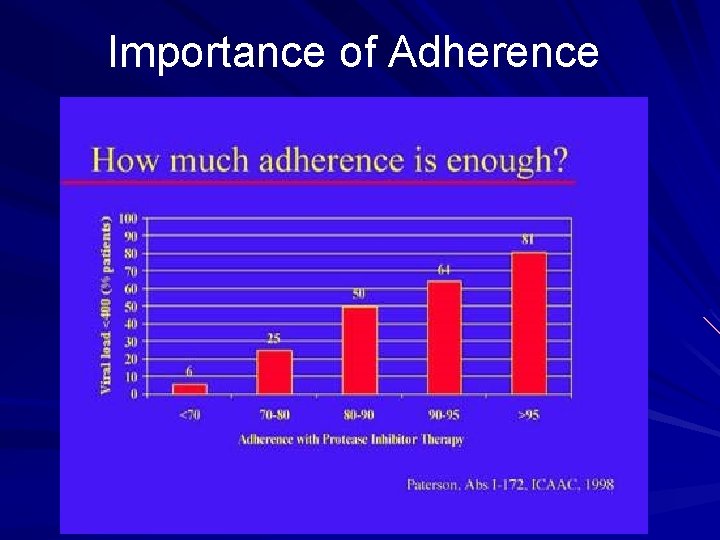
Importance of Adherence

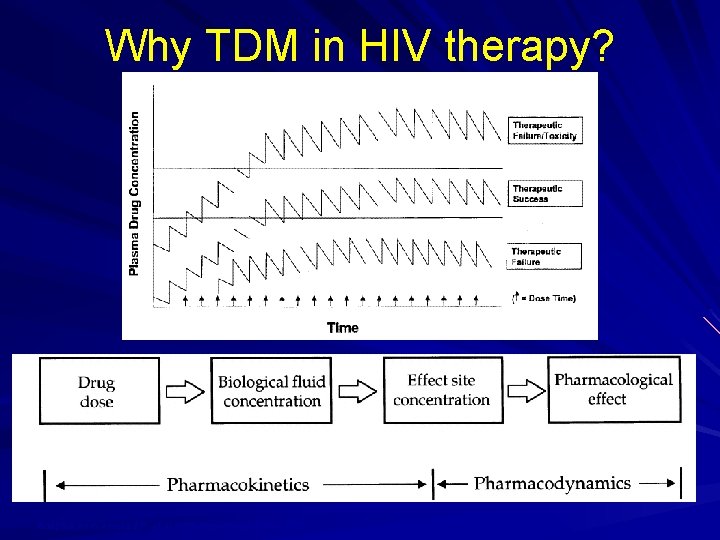
Therapeutic Drug Monitoring Not widely used in the US Recommended in certain situations for PIs and NNRTIs What makes a drug a good candidate for TDM? When should TDM be performed for antiretrovirals?

Why TDM in HIV therapy? Adapted from Acosta EP, et al AIDS Res Human Retro 2002
 Anti a anti b anti rh blood type
Anti a anti b anti rh blood type Türkçe çeviri
Türkçe çeviri Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Phosphoanhydride bonds
Phosphoanhydride bonds Orotic
Orotic Nucleoside reverse transcriptase inhibitors
Nucleoside reverse transcriptase inhibitors Kim foglia dna replication
Kim foglia dna replication Nucleoside and nucleotide
Nucleoside and nucleotide Quinolones mode of action
Quinolones mode of action Classe e subclasse dos verbos
Classe e subclasse dos verbos Pre ap classes vs regular classes
Pre ap classes vs regular classes Venipuncture radiologic technologist
Venipuncture radiologic technologist Hepatic extraction ratio formula
Hepatic extraction ratio formula Pharmacology newcastle
Pharmacology newcastle Basic principles of pharmacology
Basic principles of pharmacology Guaifenasin
Guaifenasin Chapter 30 principles of pharmacology
Chapter 30 principles of pharmacology Glomerular
Glomerular Basic & clinical pharmacology
Basic & clinical pharmacology Pharmacology definition
Pharmacology definition Pharmacology for nurses: a pathophysiological approach
Pharmacology for nurses: a pathophysiological approach Focus on pharmacology essentials for health professionals
Focus on pharmacology essentials for health professionals What is ion trapping in pharmacology
What is ion trapping in pharmacology Define pharmacology
Define pharmacology Advantages and disadvantages of drugs
Advantages and disadvantages of drugs Therapeutic index
Therapeutic index Define pharmacology
Define pharmacology Pharmacology tutor anderson
Pharmacology tutor anderson Dopamine synthesis
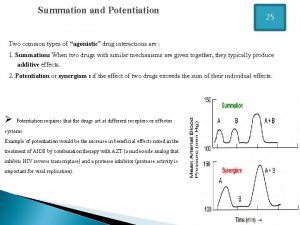
Dopamine synthesis Drug summation examples
Drug summation examples Clinical pharmacology powered by clinicalkey
Clinical pharmacology powered by clinicalkey What is pharmacology
What is pharmacology Clinical pharmacology seminar
Clinical pharmacology seminar First bypass effect
First bypass effect