Daily Anti Retroviral Therapy II Once Daily Regimen

![PLASMA CONCENTRATION [ng/m. L] d 4 T XR 100 mg Once Daily Comparable Drug PLASMA CONCENTRATION [ng/m. L] d 4 T XR 100 mg Once Daily Comparable Drug](https://slidetodoc.com/presentation_image/b89db0a635fca514c4f87f26f0461f99/image-4.jpg)






- Slides: 19

Daily Anti. Retroviral Therapy II Once Daily Regimen for Treatment-naïve HIV+ Patients with Stavudine XR + Lamivudine + Efavirenz 24 Week Interim Efficacy and Safety Results D. Jayaweera 1, S. Becker 2, F. Felizarta 3, M. Sands 4, L. Slater 5 S. Gothelf 6, J. Maa 6, C. Dezii 6, S. Hodder 6, J. Tudor 6 University of Miami, FL, USA. Pacific Horizon Medical Group, San Francisco, CA, USA. 3 34 th Street Community Health Center, Bakersfield, CA, USA. 4 University of Florida, Jacksonville, FL, USA. 5 University of Oklahoma, Oklahoma City, OK, USA. 6 Bristol-Myers Squibb, Plainsboro, , NJ, USA. Plainsboro 1 2 DART II

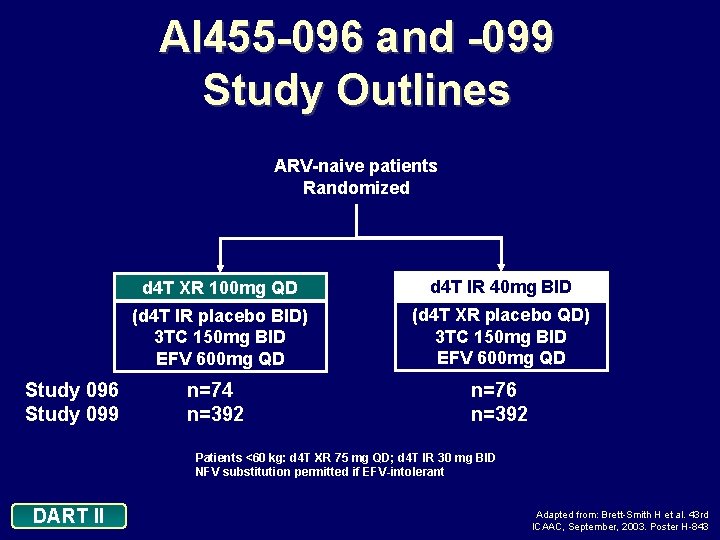
AI 455 -096 and -099 Study Outlines ARV-naive patients Randomized Study 096 Study 099 d 4 T XR 100 mg QD d 4 T IR 40 mg BID (d 4 T IR placebo BID) 3 TC 150 mg BID EFV 600 mg QD (d 4 T XR placebo QD) 3 TC 150 mg BID EFV 600 mg QD n=74 n=392 n=76 n=392 Patients <60 kg: d 4 T XR 75 mg QD; d 4 T IR 30 mg BID NFV substitution permitted if EFV-intolerant DART II Adapted from: Brett-Smith H et al. 43 rd ICAAC, September, 2003. Poster H-843

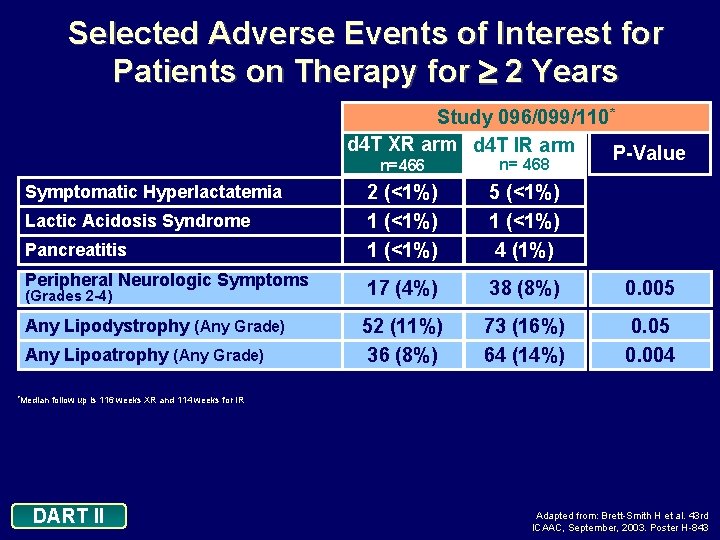
Selected Adverse Events of Interest for Patients on Therapy for 2 Years Study 096/099/110* d 4 T XR arm d 4 T IR arm P-Value Symptomatic Hyperlactatemia Lactic Acidosis Syndrome Pancreatitis Peripheral Neurologic Symptoms (Grades 2 -4) Any Lipodystrophy (Any Grade) Any Lipoatrophy (Any Grade) *Median n=466 n= 468 2 (<1%) 1 (<1%) 5 (<1%) 1 (<1%) 4 (1%) 17 (4%) 38 (8%) 0. 005 52 (11%) 36 (8%) 73 (16%) 64 (14%) 0. 05 0. 004 follow up is 116 weeks XR and 114 weeks for IR DART II Adapted from: Brett-Smith H et al. 43 rd ICAAC, September, 2003. Poster H-843
![PLASMA CONCENTRATION ngm L d 4 T XR 100 mg Once Daily Comparable Drug PLASMA CONCENTRATION [ng/m. L] d 4 T XR 100 mg Once Daily Comparable Drug](https://slidetodoc.com/presentation_image/b89db0a635fca514c4f87f26f0461f99/image-4.jpg)
PLASMA CONCENTRATION [ng/m. L] d 4 T XR 100 mg Once Daily Comparable Drug Exposure to d 4 T IR 40 mg BID Peak (Cmax) for d 4 T XR is ~50% of the IR formulation 1000 100 d 4 T XR formulation has 2 -3 times higher trough plasma levels than d 4 T IR 10 1 0 4 12 8 16 TIME [h] DART II d 4 T XR *Parallel d 4 T IR groups for XR and IR formulations in HIV patients 20 24

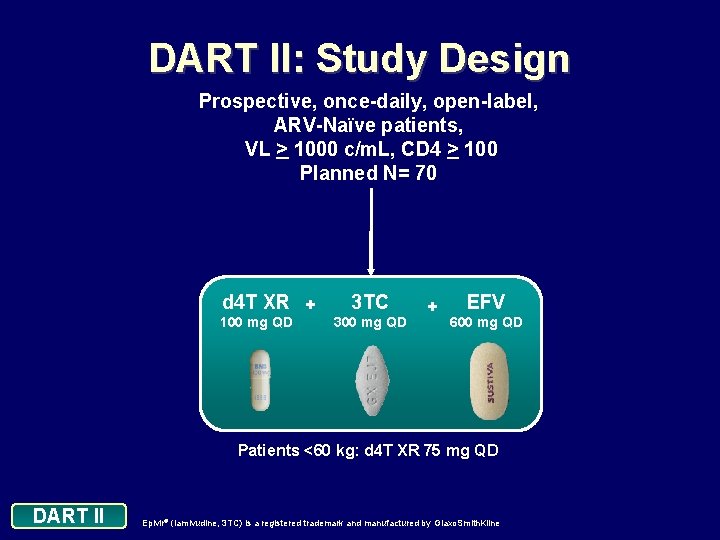
DART II: Study Design Prospective, once-daily, open-label, ARV-Naïve patients, VL > 1000 c/m. L, CD 4 > 100 Planned N= 70 d 4 T XR + 100 mg QD 3 TC 300 mg QD + EFV 600 mg QD Patients <60 kg: d 4 T XR 75 mg QD DART II Epivir® (lamivudine, 3 TC) is a registered trademark and manufactured by Glaxo. Smith. Kline

DART II: OBJECTIVES Primary Objective: • Efficacy of once daily d 4 T XR + 3 TC + EFV as determined by proportion of patients with plasma HIV-RNA <400 copies/m. L at 48 -weeks Secondary Objective: • Proportion of patients with plasma HIV-RNA <400 and <50 copies/m. L at Weeks 24, 48, 72, and 96 • Patient adherence using pill counts and Antiviral Medication Adherence Form (AMAF) • Safety and tolerability DART II

Baseline Characteristics d 4 T XR QD 3 TC QD + EFV QD (N=64) Median Age, (years) Demography Male 83% White 42% Black 45% Other 13% Median HIV RNA (log 10 copies/m. L) Clinical 100, 000 copies/m. L Median CD 4 Counts (cells/ L) DART II 37 4. 7 30% 315

Patient Disposition at Week 24 d 4 T XR QD 3 TC QD + EFV QD Evaluable Total Discontinuations, n (%) 64 10 (16%) Reasons for Discontinuation DART II Adverse Event 3 (5%) Withdrew Consent 4 (6%) Lost to Follow-up 2 (3%) Virologic Failure 1 (2%) Serious Adverse Event 0 Death 0

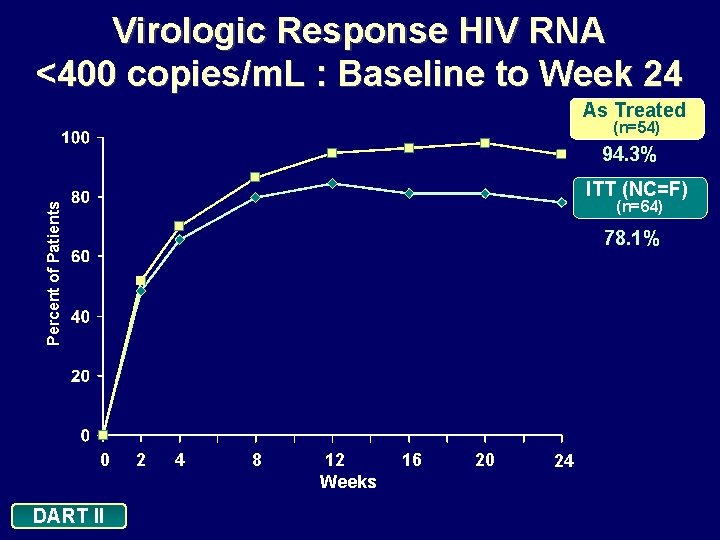
Virologic Response HIV RNA <400 copies/m. L : Baseline to Week 24 As Treated (n=54) 94. 3% ITT (NC=F) Percent of Patients (n=64) 78. 1% 0 DART II 2 4 8 12 Weeks 16 20 24

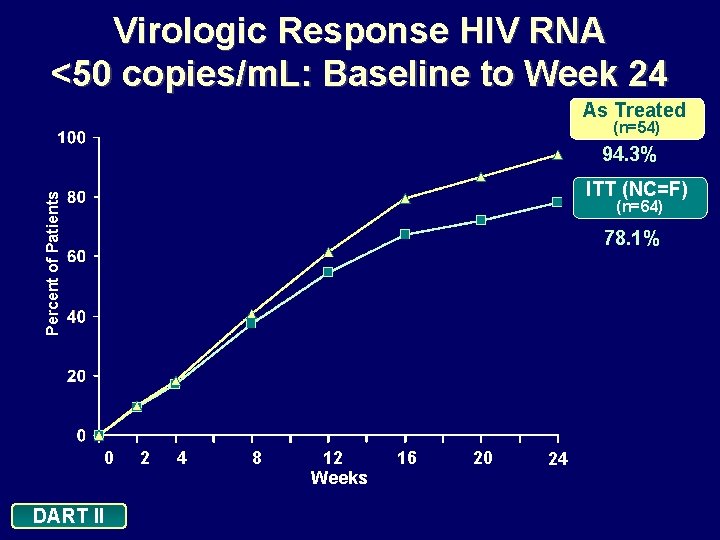
Virologic Response HIV RNA <50 copies/m. L: Baseline to Week 24 As Treated (n=54) 94. 3% Percent of Patients ITT (NC=F) (n=64) 78. 1% 0 DART II 2 4 8 12 Weeks 16 20 24

HIV RNA Change (log 10 copies/m. L) Mean Change in HIV RNA Level: Baseline to Week 24 0 -0. 5 -1 -1. 5 -2 - 2. 7 -2. 5 -3 DART II 0 2 4 8 12 16 Weeks 20 24

CD 4 Cell Mean Change: Baseline to Week 24 CD 4 Mean Change (cells/mm 3) 160 140 120 100 80 CD 4 cell count Baseline: 354 Week 24: 488 60 40 20 0 0 DART II 2 4 8 12 16 Weeks 20 24

Grade 2 - 4 Treatment Related Adverse Events (>2%) Grade 2 -4 d 4 T XR + 3 TC + EFV n=64 Nausea 4 (6. 3%) Hypoaesthesia 3 (4. 7%) Peripheral Neuropathy 2 (3. 1%) Diarrhea 2 (3. 1%) Somnolence 2 (3. 1%) Vomiting 2 (3. 1%) DART II

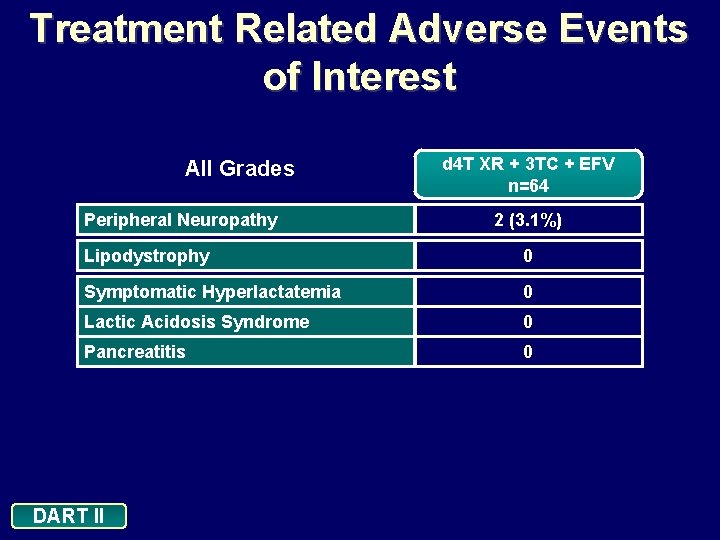
Treatment Related Adverse Events of Interest All Grades Peripheral Neuropathy d 4 T XR + 3 TC + EFV n=64 2 (3. 1%) Lipodystrophy 0 Symptomatic Hyperlactatemia 0 Lactic Acidosis Syndrome 0 Pancreatitis 0 DART II

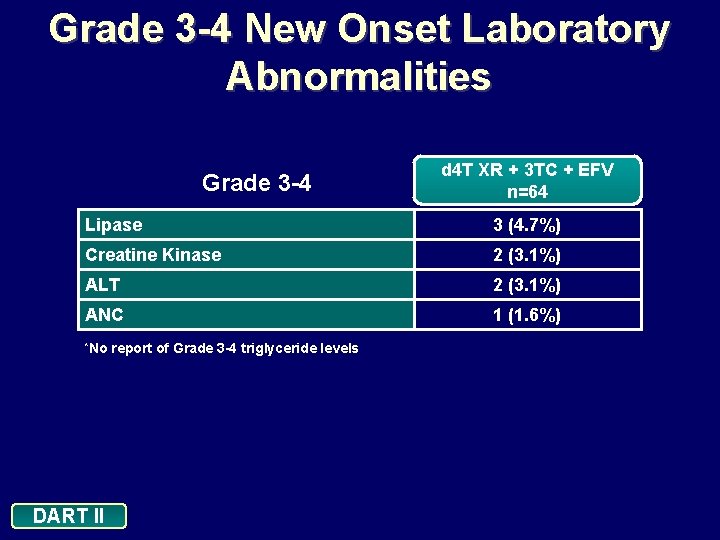
Grade 3 -4 New Onset Laboratory Abnormalities Grade 3 -4 d 4 T XR + 3 TC + EFV n=64 Lipase 3 (4. 7%) Creatine Kinase 2 (3. 1%) ALT 2 (3. 1%) ANC 1 (1. 6%) *No DART II report of Grade 3 -4 triglyceride levels

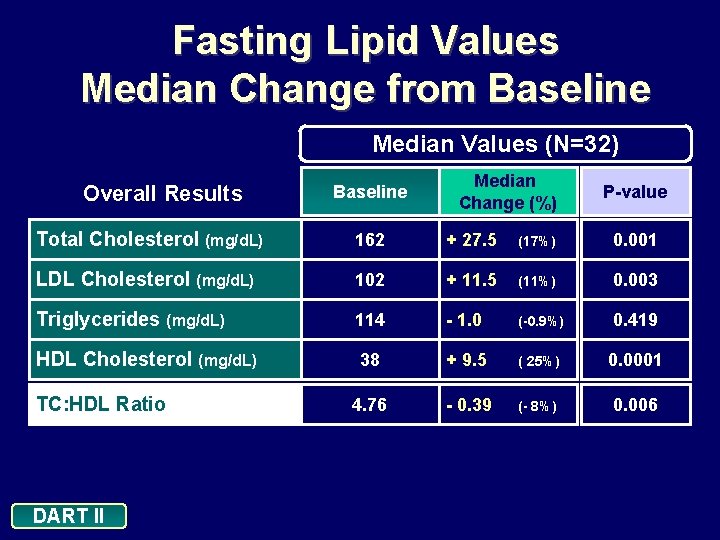
Fasting Lipid Values Median Change from Baseline Median Values (N=32) Overall Results Baseline Median Change (%) P-value Total Cholesterol (mg/d. L) 162 + 27. 5 (17%) 0. 001 LDL Cholesterol (mg/d. L) 102 + 11. 5 (11%) 0. 003 Triglycerides (mg/d. L) 114 - 1. 0 (-0. 9%) 0. 419 HDL Cholesterol (mg/d. L) 38 + 9. 5 ( 25%) 0. 0001 4. 76 - 0. 39 (- 8%) 0. 006 TC: HDL Ratio DART II

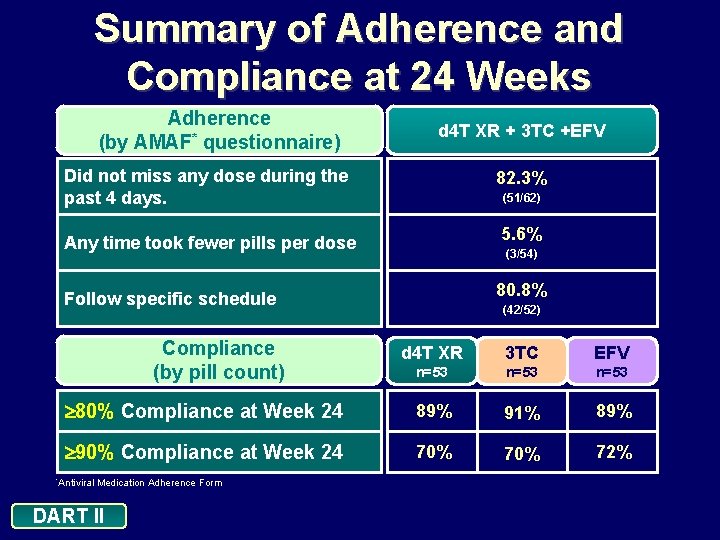
Summary of Adherence and Compliance at 24 Weeks Adherence (by AMAF* questionnaire) d 4 T XR + 3 TC +EFV Did not miss any dose during the past 4 days. 82. 3% Any time took fewer pills per dose 5. 6% Follow specific schedule 80. 8% Compliance (by pill count) (51/62) (3/54) (42/52) d 4 T XR 3 TC EFV n=53 80% Compliance at Week 24 89% 91% 89% 90% Compliance at Week 24 70% 72% *Antiviral Medication Adherence Form DART II

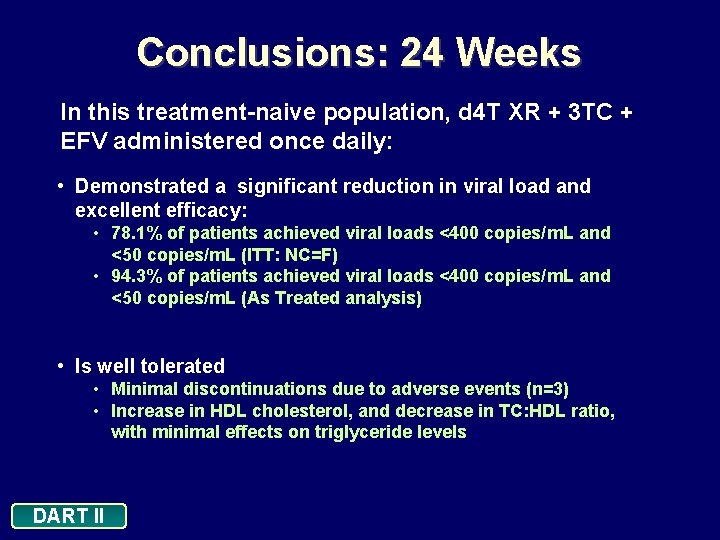
Conclusions: 24 Weeks In this treatment-naive population, d 4 T XR + 3 TC + EFV administered once daily: • Demonstrated a significant reduction in viral load and excellent efficacy: • 78. 1% of patients achieved viral loads <400 copies/m. L and <50 copies/m. L (ITT: NC=F) • 94. 3% of patients achieved viral loads <400 copies/m. L and <50 copies/m. L (As Treated analysis) • Is well tolerated • Minimal discontinuations due to adverse events (n=3) • Increase in HDL cholesterol, and decrease in TC: HDL ratio, with minimal effects on triglyceride levels DART II

Acknowledgments TO ALL THE PATIENTS AND STUDY CENTER PARTICIPANTS Stephen Becker, MD. San Francisco, CA Coordinator: Sunita Lundy Gary Richmond, MD. Ft. Lauderdale, FL Coordinator: Vernon Appleby, RN Nicholaos Bellos, MD. Dallas, TX Coordinators: Christopher Hayes Dawn Chaney Michael Sands, MD. Jacksonville, FL Coordinator: Debbie Aragon, RN Paul Cook, MD. Greenville, NC Coordinator: Grace Wilkins, RN Kunthavi Sathasivam, MD. Washington, DC Coordinator: Takada Harris Franco Felizarta, MD. Bakersfield, CA Coordinator: Jennifer Kuhach Leonard Slater, MD. Oklahoma City, OK Coordinator: Brenda Verel, LPN Dushyantha Jayaweera, MD. Miami, FL Coordinator: Rose Lalanne, RN DART II
 Anti a anti b anti rh blood type
Anti a anti b anti rh blood type Türkçe çeviri
Türkçe çeviri Anti impulse therapy
Anti impulse therapy Bioness bits cost
Bioness bits cost What are the major humanistic therapies
What are the major humanistic therapies Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Ageloc body shaping gel regimen
Ageloc body shaping gel regimen Rockey davis incision
Rockey davis incision Regimen hibrido
Regimen hibrido Beers criteria 2021 pocket card
Beers criteria 2021 pocket card Que es regimen general
Que es regimen general Regimen exportacion
Regimen exportacion Emaco regimen
Emaco regimen Comercio en el antiguo regimen
Comercio en el antiguo regimen Pid opd regimen
Pid opd regimen Unipartidismo en china
Unipartidismo en china Ochsner sherren regime
Ochsner sherren regime Regimen patrimonial del estado
Regimen patrimonial del estado Subordinada sustantiva
Subordinada sustantiva Crv sintaxis
Crv sintaxis