Analytical quality control AQC for pesticide residues analysis


















- Slides: 24

Analytical quality control (AQC) for pesticide residues analysis Alan Hill Central Science Laboratory, York, UK Rome, 26 -28 November, 2001

Why is AQC necessary? • Analysis is complex – many things can go wrong – interpretation of results can be difficult. • Provides evidence to support results – supports the credibility of data.

SANCO/3013/2000 • Covers all aspects of AQC. • Objectives: – avoid false positives and false negatives; – acceptable accuracy and precision; – EU harmonisation of fit-for-purpose AQC, to provide uniform level of confidence in reported data.

General issues relating to topics that other speakers will consider… • Accreditation, record keeping, traceability, etc. – important to document the supporting evidence. • Sampling and sub-sampling – important because these procedures determine the actual residue levels present, irrespective of the quality of the analysis.

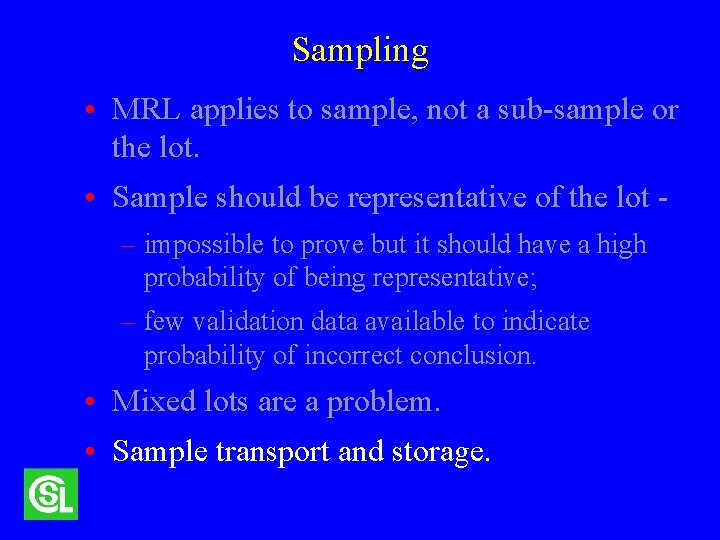
Sampling • MRL applies to the sample, not a subsample or the lot. • Sample should be representative of the lot – impossible to prove but it should have a high probability of being representative; – few validation data available to show the probability of an incorrect conclusion. • Mixed lots are a problem.

What is a uniform lot?

Sampling • MRL applies to sample, not a sub-sample or the lot. • Sample should be representative of the lot – impossible to prove but it should have a high probability of being representative; – few validation data available to indicate probability of incorrect conclusion. • Mixed lots are a problem. • Sample transport and storage.

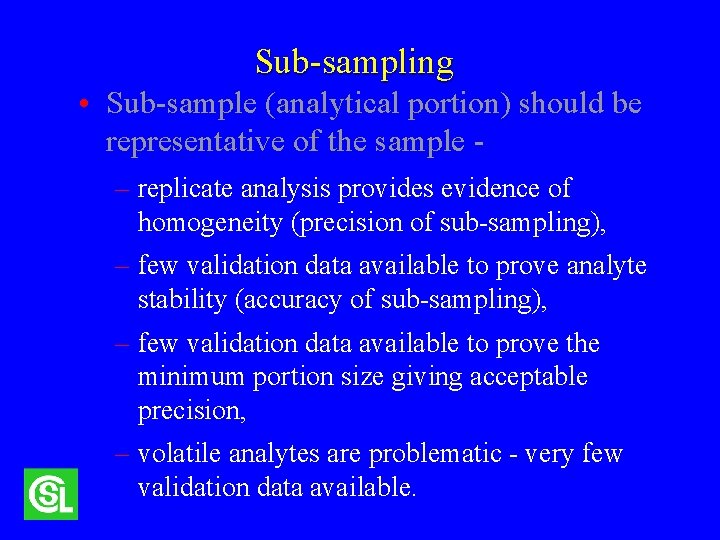
Sub-sampling • Sub-sample (analytical portion) should be representative of the sample -

Sub-sampling

Sub-sampling • Sub-sample (analytical portion) should be representative of the sample – replicate analysis provides evidence of homogeneity (precision of sub-sampling), – few validation data available to prove analyte stability (accuracy of sub-sampling), – few validation data available to prove the minimum portion size giving acceptable precision, – volatile analytes are problematic - very few validation data available.

Calibration standards • Accuracy is dependent on good standards. • Standards occasionally incorrectly identified. • Purity can be difficult to determine, hence expiry dates. • Cross-checking “new” and “old” can help to eliminate gross errors.

Extraction & concentration • Very few validation data for extraction efficiency – not usually a problem with fruit and vegetables but may be with dry cereal products. • Could be an issue where a common moiety is determined. • Precision requirements may restrict the use of small extract volumes. • Internal standards may have advantages.

Contamination and interference • Can occur in the best laboratories. • Leads to poor accuracy, wrong identification, no results and/or higher costs. • Potential contamination with the analyte can be detected by use of mixed standards. • Interference can be indirect (“matrix effects”). • Natural presence of the analyte - a problem.

Calibration • Shape of detector response curve must be known. • Changes in detector response must be tracked. • Number of points and frequency of repeat injections dependent upon stability of detector response. • Extrapolation of the calibration curve beyond LCL and HCL requires caution.

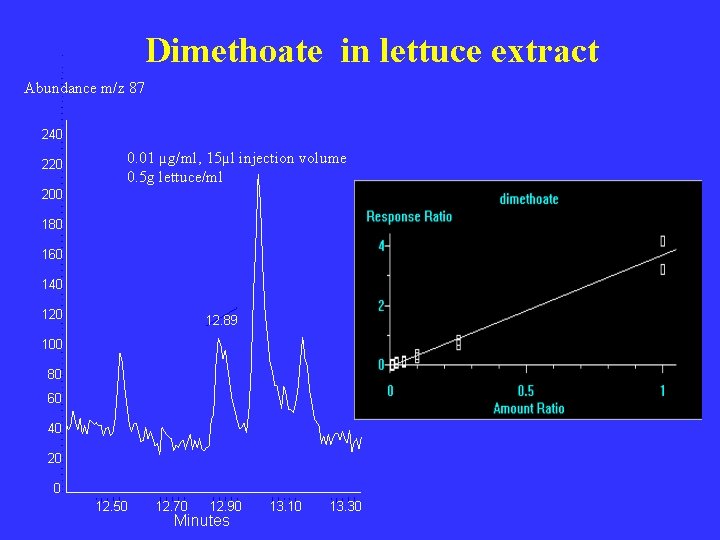
Dimethoate in lettuce extract Abundance m/z 87 240 220 0. 01 µg/ml, 15µl injection volume 0. 5 g lettuce/ml 200 180 160 140 12. 89 100 80 60 40 20 0 12. 50 12. 70 12. 90 Minutes 13. 10 13. 30

Calibration • “Matrix effects” should be taken into account. • “Matrix effects” are variable: the only way to be sure of compensating for them is by “standard addition”. • “Representative analytes” may be used but data must be interpreted with caution.

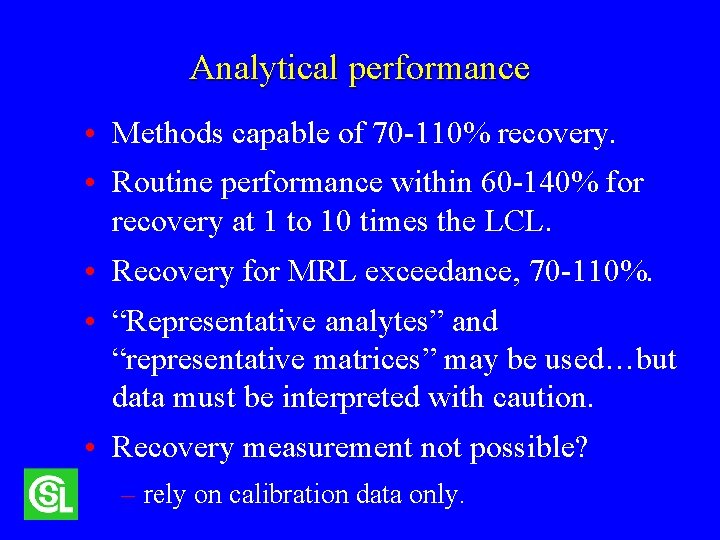
Analytical performance • Methods capable of 70 -110% recovery. • Routine performance within 60 -140% for recovery at 1 to 10 times the LCL. • Recovery for MRL exceedance, 70 -110%. • “Representative analytes” and “representative matrices” may be used…but data must be interpreted with caution. • Recovery measurement not possible? – rely on calibration data only.

Analytical performance • Participate in proficiency tests. • Investigate unacceptable scores and rectify problems. • Analyse in-house reference materials – mainly suitable for single-analyte methods.

Confirmation • Negative results require acceptable LCL and recovery. • Positive results require acceptable calibration and recovery and… – other requirements decided case-by-case… – should be qualitative and quantitative; – use different detectors, columns etc; – MS techniques particularly important.

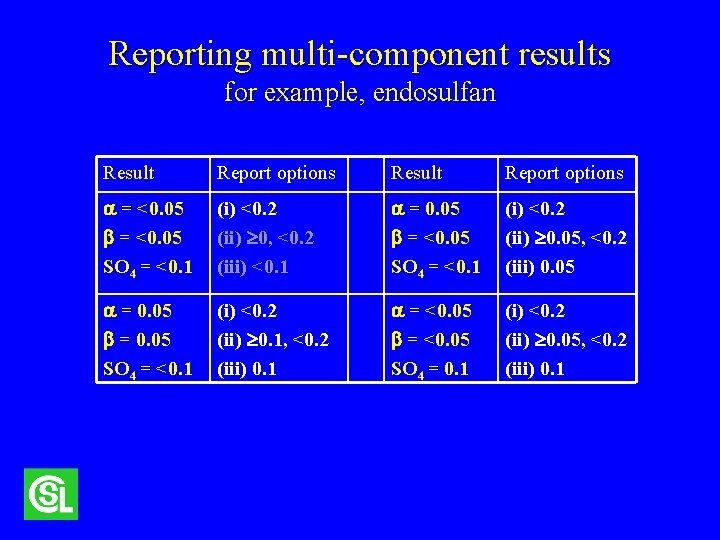
Reporting multi-component results for example, endosulfan Result Report options = <0. 05 SO 4 = <0. 1 (i) <0. 2 (ii) 0, <0. 2 (iii) <0. 1 = 0. 05 = <0. 05 SO 4 = <0. 1 (i) <0. 2 (ii) 0. 05, <0. 2 (iii) 0. 05 = 0. 05 SO 4 = <0. 1 (i) <0. 2 (ii) 0. 1, <0. 2 (iii) 0. 1 = <0. 05 SO 4 = 0. 1 (i) <0. 2 (ii) 0. 05, <0. 2 (iii) 0. 1

Uncertainty • Required by ISO 17025. • “Typical uncertainty”, not specific uncertainty. • Most easily measured from AQC data, precision and accuracy required. • Doubts remain over the best methods for estimation. • Users of data may not understand the implications.

In future…. . ? • • Cost reduction is important…. . AQC for 0. 01 -0. 05 mg/kg residues? AQC for rapid or large-scale screening? In both cases: – must avoid major bias and – must have correct identification but… – what precision is acceptable? what range for routine recovery? – what limits for ion ratios for confirmation?

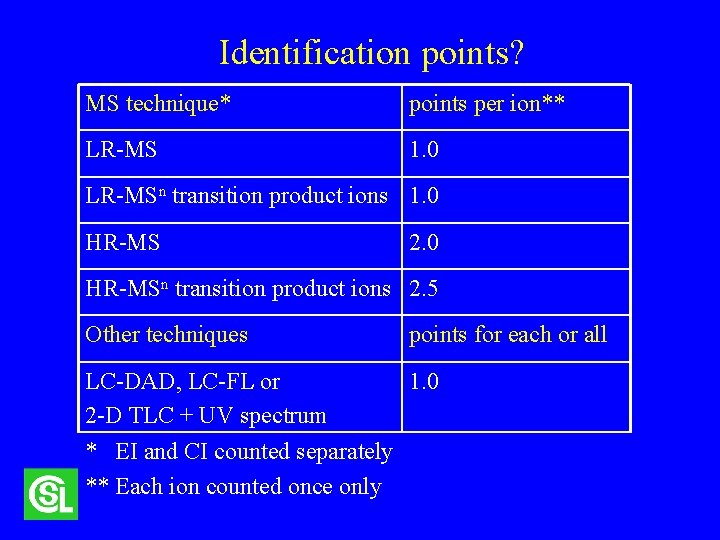
Identification points? MS technique* points per ion** LR-MS 1. 0 LR-MSn transition product ions 1. 0 HR-MS 2. 0 HR-MSn transition product ions 2. 5 Other techniques points for each or all LC-DAD, LC-FL or 1. 0 2 -D TLC + UV spectrum * EI and CI counted separately ** Each ion counted once only

Summary • AQC provides the evidence to support validity of results. • More rigorous AQC = more support, • Less rigorous AQC = less support. • Results and AQC must be fit for purpose. • The analyst or the “customer” must decide fitness for purpose but… • …if a result is proven to be wrong, the analyst will blamed!
 Aqc quality control
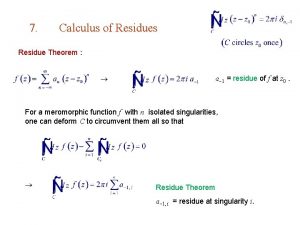
Aqc quality control Residue theorem
Residue theorem Perform quality assurance
Perform quality assurance Pmp quality management
Pmp quality management Pmbok quality management
Pmbok quality management Concept of quality assurance
Concept of quality assurance Pesticide resistance
Pesticide resistance Pesticide resistance
Pesticide resistance Lanate pesticide
Lanate pesticide Pesticide classification chart
Pesticide classification chart Herbicide license ontario
Herbicide license ontario Agriculture pesticide difenoconazole
Agriculture pesticide difenoconazole Virginia pesticide registration
Virginia pesticide registration Wisconsin pesticide applicator license
Wisconsin pesticide applicator license Tablica miješanja pesticida
Tablica miješanja pesticida Msg pesticide
Msg pesticide Pesticide educational resources collaborative
Pesticide educational resources collaborative European pesticide residue workshop
European pesticide residue workshop Types of pesticides
Types of pesticides Brewery quality control
Brewery quality control Process of nursing audit
Process of nursing audit Quality improvement vs quality assurance
Quality improvement vs quality assurance Philip crosby formulated the seven deadly diseases
Philip crosby formulated the seven deadly diseases Quality is free
Quality is free Old quality vs new quality
Old quality vs new quality