Air Pressure Properties of Air Air contains atoms











- Slides: 13

Air Pressure

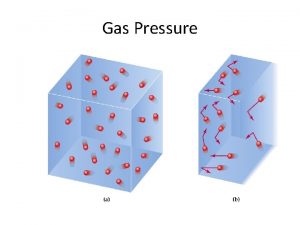
Properties of Air § Air contains atoms and molecules that have mass, so air has mass as well. Since air has mass, it also has other properties like density and pressure. § Density – Density is the amount of mass in a given volume (d=m/v). – the greater mass, the greater the density.

Pressure – Pressure is the force exerted in a given area. – Air pressure is the result of the weight of a column of air pushing down on an area. The molecules in air push in all directions and that is why air pressure does not crush objects. – Denser air exerts more pressure than less dense air.

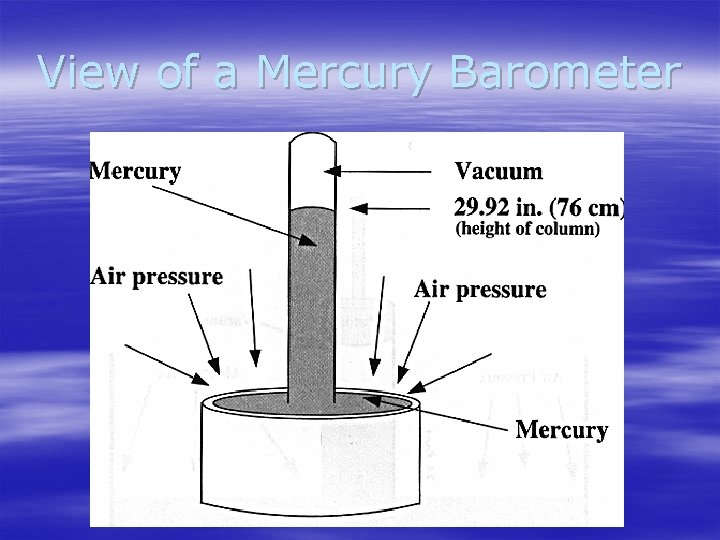
Measuring Air Pressure § A barometer is an instrument used to measure air pressure. The most common are mercury barometer and aneroid barometers. § Mercury barometer – Consists of a glass tube open at the bottom end and partially filled with mercury. The open end rests in a dish of mercury and the space in the tube above the mercury is a vacuum. – The air pressure that pushes down on the mercury in the dish is equal to the weight of the column of mercury in the tube. At sea level, the column is on average 76 cm high.

View of a Mercury Barometer

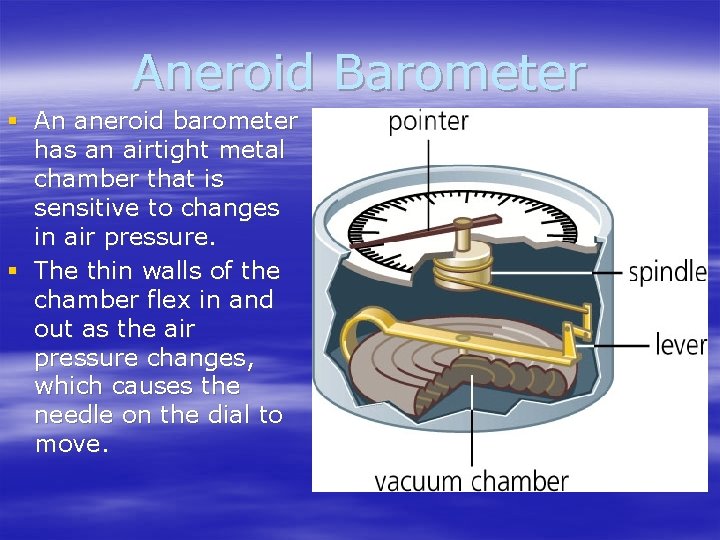
Aneroid Barometer § An aneroid barometer has an airtight metal chamber that is sensitive to changes in air pressure. § The thin walls of the chamber flex in and out as the air pressure changes, which causes the needle on the dial to move.

1. Define the terms weather and atmosphere. 2. Why is the abundance of a gas in the atmosphere usually shown as a percentage of “dry air”? 3. In decreasing order, what are the four most abundant gases found in “dry air” on earth’s atmosphere? 4. What are two different processes that rely on oxygen? In which two forms (please include chemical formulas) is oxygen found in Earth’s atmosphere?


Units of Air Pressure § In weather reports, air pressure is usually given in inches of mercury. National Weather Service maps use millibars. § One inch of mercury equals 33. 87 millibars. (25 = 846. 75)

Altitude and Air Pressure § Altitude, or elevation, is the distance above sea level. As altitude increases, air pressure decreases. As air pressure decreases, so does density. § Air at sea level has the weight of the whole atmosphere pushing down on it so air pressure at sea level is the highest. § At the top of a mountain the air pressure is less and so is air’s density. This is why it is difficult to breathe, since there is less oxygen in each cubic meter of air.

5. How would the amount of carbon dioxide in earth’s atmosphere change if there were no plants? 6. How does water vapor in Earth’s atmosphere relate to weather? 7. Describe three ways in which Earth’s atmosphere makes the Earth habitable for living organisms. 8. Why does air exert pressure? What causes there to be air pressure in Earth’s atmosphere?

9. What two instruments are typically used to measure air pressure? 10. What two different units of measure are commonly used to measure air pressure? 11. One inch of mercury is equal to how many millibars? Therefore, 29. 5 inches of mercury would equal how many millibars?

12. How are air pressure and air density affected as altitude increases? Why does this happen? 13. Is air pressure greater at sea level (0 feet of elevation), or on the top of Mount Everest (29, 029 feet)? Why? 14. Why do commercial airlines, which typically fly at a cruising altitude of 35, 000 feet, need to keep the cabins pressurized?
 Regents periodic table
Regents periodic table Properties of atoms and the periodic table
Properties of atoms and the periodic table Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chapter 2 life's chemical basis
Chapter 2 life's chemical basis Air temperature
Air temperature Air mass vocabulary
Air mass vocabulary Pressure tolerant vs pressure sensitive
Pressure tolerant vs pressure sensitive Pressure support vs pressure control
Pressure support vs pressure control Pressure mapping for pressure ulcers
Pressure mapping for pressure ulcers Intrapleural pressure
Intrapleural pressure Starling forces equation
Starling forces equation Partial pressure formula
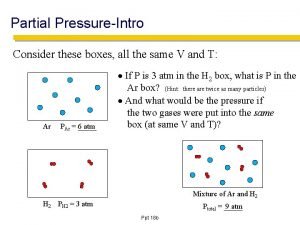
Partial pressure formula