Aim How to write nuclear reactions Nuclear Energy







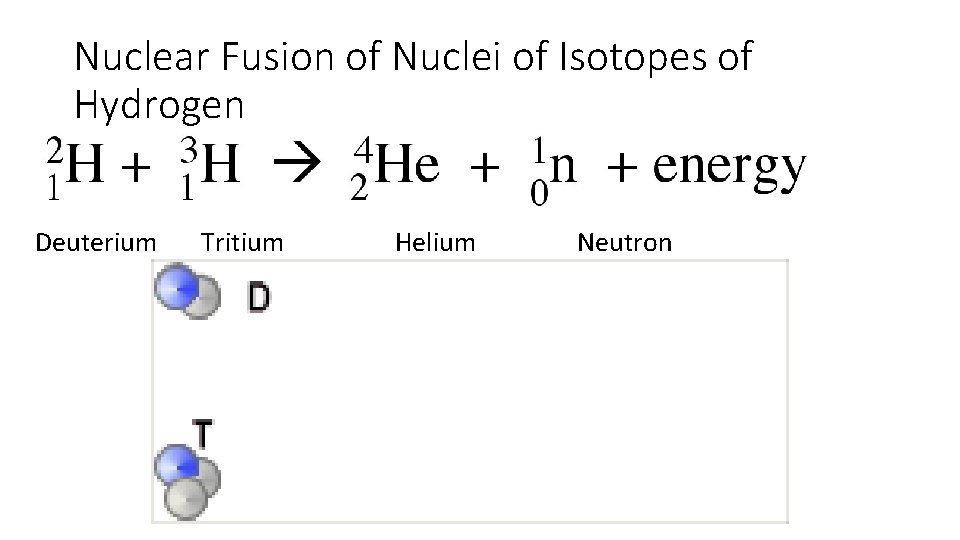

- Slides: 14

Aim: How to write nuclear reactions?

Nuclear Energy • In nuclear reaction, mass is converted into energy; there is no conservation of mass.

Natural Transmutations (Spontaneous) • Alpha, Beta, and Positron decay are natural transmutations that occur because of unstable nuclei (neutron: proton ratio not 1: 1)

Artificial Transmutations (Nonspontaneous) • When scientist collide a charged particle with a nucleus, to create new nuclei • The second type involves the collision of a neutron and a nucleus. Used in nuclear reactors to prepare radioactive nuclei from stable nuclei. • Easy to tell the difference between natural and artificial transmutation, artificial transmutation will have two reactants.



Nuclear Fission • Occurs when one atom absorbs a neutron and splits into two or more pieces, giving off tremendous amount of energy. • In a fission reaction, the sum of the masses of the pieces formed is less than the mass of the original heavier piece, because some mass is converted into energy.

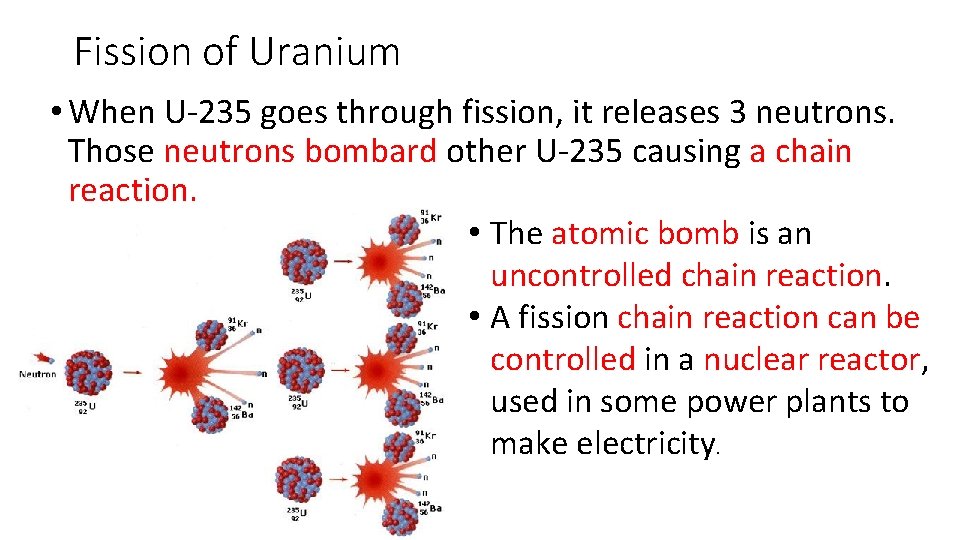
Fission of Uranium • When U-235 goes through fission, it releases 3 neutrons. Those neutrons bombard other U-235 causing a chain reaction. • The atomic bomb is an uncontrolled chain reaction. • A fission chain reaction can be controlled in a nuclear reactor, used in some power plants to make electricity.

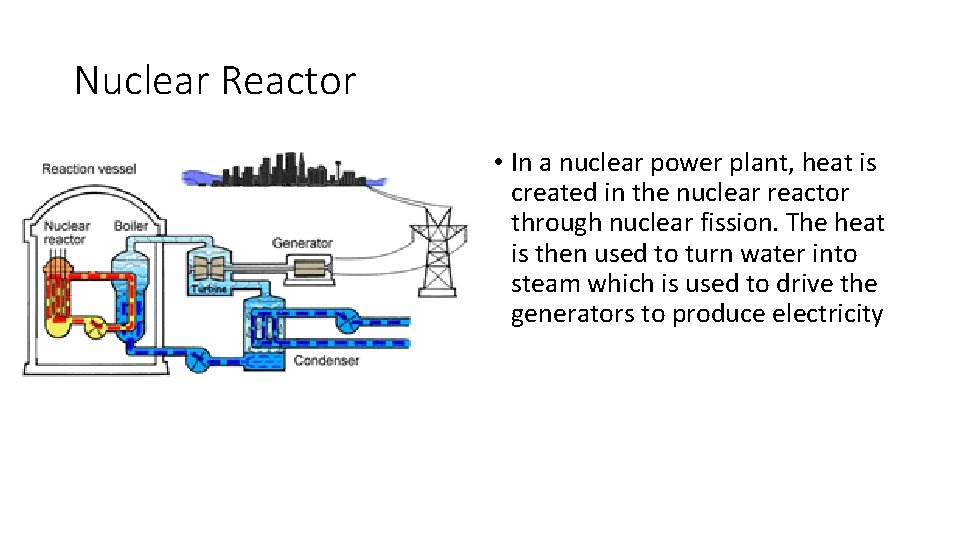
Nuclear Reactor • In a nuclear power plant, heat is created in the nuclear reactor through nuclear fission. The heat is then used to turn water into steam which is used to drive the generators to produce electricity

Nuclear Fusion • Nuclear fusion is the joining (or fusing) of the nuclei of two atoms to form a single heavier atom. • At extremely high temperatures and high pressure, the nuclei (positively charged) can readily combine to form heavier elements and in the process release considerable energy. • This reaction occurs on the sun.

Nuclear Fusion • The mass of the nucleus formed after fusion is less than the sum of the mass of the two substances fused, because some mass is converted to energy. • The energy generated in a fusion reaction is greater than the energy generation in a fission reaction.
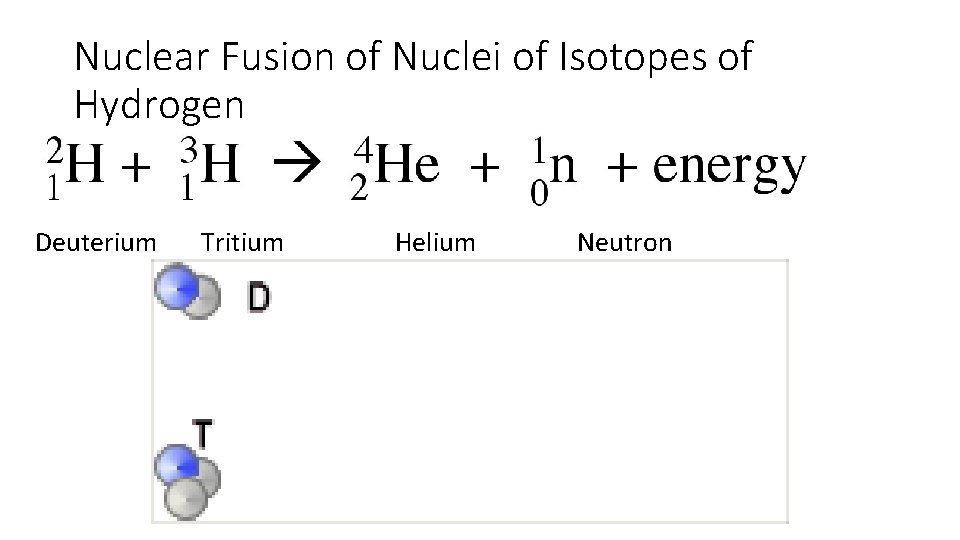
Nuclear Fusion of Nuclei of Isotopes of Hydrogen Deuterium Tritium Helium Neutron

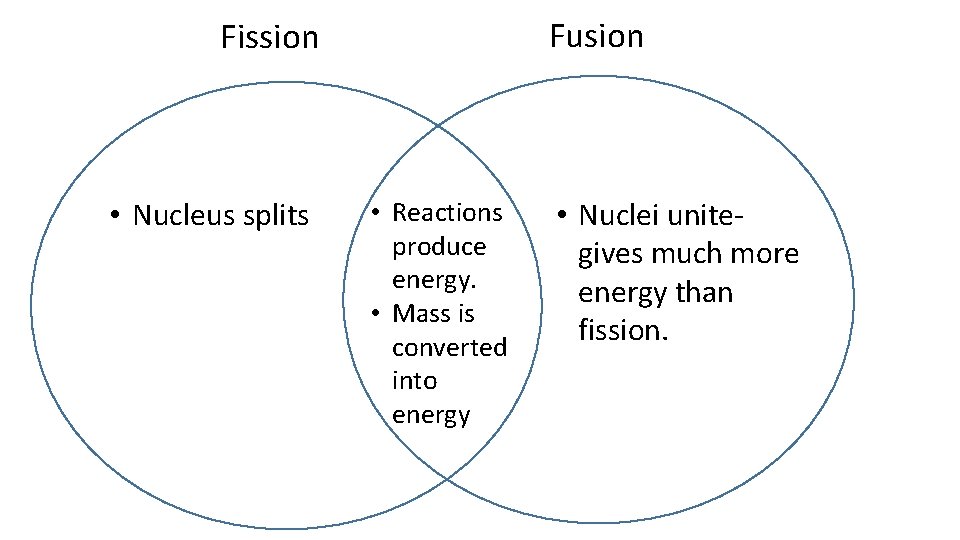
Fusion Fission • Nucleus splits • Reactions produce energy. • Mass is converted into energy • Nuclei unitegives much more energy than fission.

Radioactive Isotopes Benefits Risks 1. Tracers 2. Medical: radioactive iodine can be used to diagnose and treat thyroid. 3. Food can be stored longer. 4. Nuclear Power 5. Radioactive dating 1. Biological Damage: damage or destroy cells 2. Long Term Storage 3. Accidents: nuclear explosion 4. Pollution: nuclear waste 1. U-238 and Pb-206 are used for geological dating. 2. C-14 used for dating living material.