Addressing and Overcoming Vaccine Hesitancy William Atkinson MD













































- Slides: 56

Addressing and Overcoming Vaccine Hesitancy William Atkinson, MD, MPH* Boise Immunization Summit September 30, 2013 *Representing the Immunization Action Coalition, Saint Paul, MN

Disclosures • The speaker has no financial interest or conflict with the manufacturer of any product named in this presentation • The speaker will not discuss the off-label use of any vaccine • The speaker will not discuss vaccines not currently licensed by the Food and Drug Administration 2


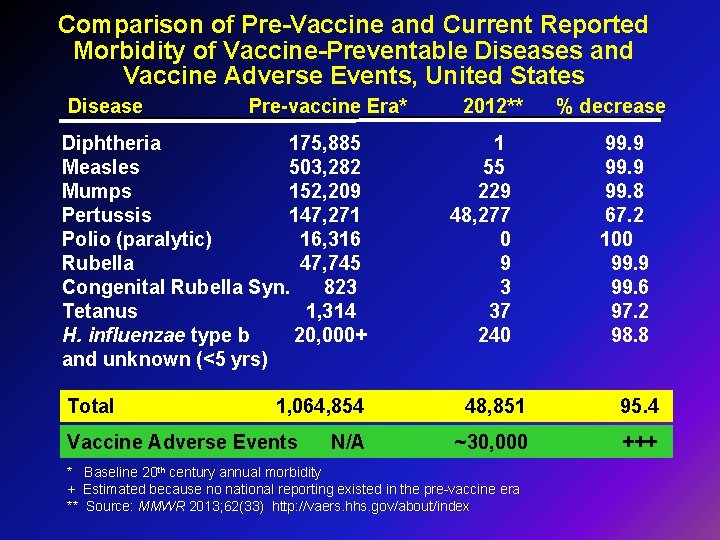
Comparison of Pre-Vaccine and Current Reported Morbidity of Vaccine-Preventable Diseases and Vaccine Adverse Events, United States Disease Pre-vaccine Era* Diphtheria 175, 885 Measles 503, 282 Mumps 152, 209 Pertussis 147, 271 Polio (paralytic) 16, 316 Rubella 47, 745 Congenital Rubella Syn. 823 Tetanus 1, 314 H. influenzae type b 20, 000+ and unknown (<5 yrs) Total 1, 064, 854 Vaccine Adverse Events N/A 2012** 1 55 229 48, 277 0 9 3 37 240 % decrease 99. 9 99. 8 67. 2 100 99. 9 99. 6 97. 2 98. 8 48, 851 95. 4 ~30, 000 +++ * Baseline 20 th century annual morbidity + Estimated because no national reporting existed in the pre-vaccine era ** Source: MMWR 2013; 62(33) http: //vaers. hhs. gov/about/index

Vaccine-Preventable Diseases Eliminated from the United States Disease • Smallpox • Polio • Measles • Rubella Last Case* 1949 1979 2000 2004 *Indigenous case. Importations may occur except for smallpox, which was eradicated from the planet in 1977 5


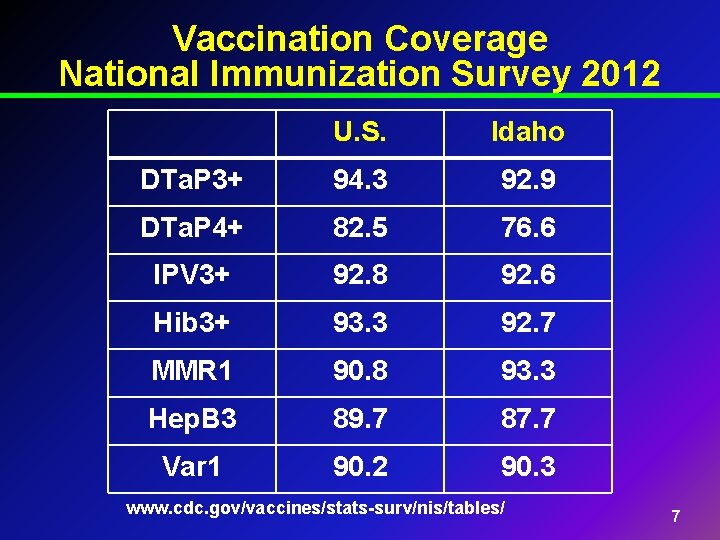
Vaccination Coverage National Immunization Survey 2012 U. S. Idaho DTa. P 3+ 94. 3 92. 9 DTa. P 4+ 82. 5 76. 6 IPV 3+ 92. 8 92. 6 Hib 3+ 93. 3 92. 7 MMR 1 90. 8 93. 3 Hep. B 3 89. 7 87. 7 Var 1 90. 2 90. 3 www. cdc. gov/vaccines/stats-surv/nis/tables/ 7

Causes of Parent/Guardian Vaccine Hesitancy • Risk versus Benefit - risk: side effects, against personal, religious, or political beliefs - benefit: protection from vaccinepreventable diseases • Most only see risk • No threat from disease 8

Causes of Parent/Guardian Vaccine Hesitancy • “Lifestyle” issues • Political issues • Fear of side effects 9




Homeopathy • Conceived by a German physician in the • • late 1700 s Originally involved the “law of similars“ - symptoms of disease can be cured by extremely small amounts of substances that produce similar symptoms in healthy people when administered in large amounts Products are extremely diluted and generally contain no active ingredient www. quackwatch. org/01 Quackery. Related. Topics/homeo. html 13

Causes of Parent/Guardian Vaccine Hesitancy • “Lifestyle” issues • Political issues • Fear of side effects 14

Causes of Parent/Guardian Vaccine Hesitancy • “Lifestyle” issues • Political issues • Fear of side effects - real or imagined 15

Importance of Vaccine Safety • Decreases in disease risks and increased attention on vaccine risks • Public confidence in vaccine safety is critical - higher standard of safety is expected of vaccines - vaccinees generally healthy (vs. ill for drugs) - lower risk tolerance results in the need to search for rare reactions 16

Importance of Vaccine Safety • Vaccinations universally recommended or mandated • Ongoing safety monitoring needed for the development of sound policies and recommendations 17

Pre-Licensure Vaccine Safety Studies • Laboratory • Animals • Humans 18

Prelicensure Human Studies • Phases I, III trials • Phase III trials usually include a control group who receive a placebo • Common reactions are identified • Most Phase III trials include 2, 000 to 5, 000 participants • Largest recent Phase III trial was REST – more than 68, 000 children 19

Postlicensure Surveillance • Identify rare reactions • Monitor increases in known reactions • Identify risk factors for reactions • Identify vaccine lots with increased rates of reactions • Identify “signals” – reports of adverse events more numerous than would be expected 20

Vaccine Adverse Event Reporting System (VAERS) • Jointly administered by CDC and FDA • National reporting system • Passive - depends on healthcare providers • and others to report Receives ~30, 000 reports per year http: //vaers. hhs. gov/ 21

Vaccine Adverse Event Reporting System (VAERS) • Detects: - new or rare events - increases in rates of known events - patient risk factors • VAERS cannot establish causality - additional studies required to confirm VAERS signals and causality • Not all reports of adverse events are causally related to vaccine 22

Post hoc ergo propter hoc “After this therefore because of this” Temporal association does not prove causation Just because one event follows another does not mean that the first caused the second 23

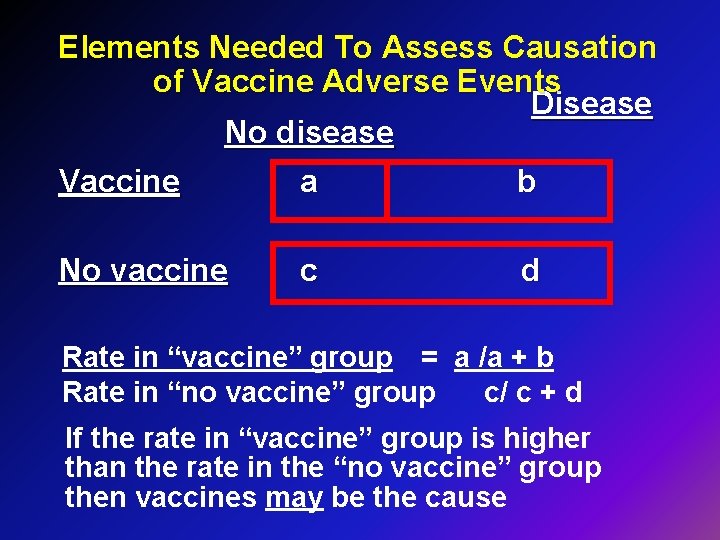
Elements Needed To Assess Causation of Vaccine Adverse Events Disease No disease Vaccine a b No vaccine c d Rate in “vaccine” group = a /a + b Rate in “no vaccine” group c/ c + d If the rate in “vaccine” group is higher than the rate in the “no vaccine” group then vaccines may be the cause

Risk of Autism Spectrum Disorder (ASD) Among Children in Denmark, 1991 -1998 Vaccine No ASD 345 No vaccine 77 ASD 440, 310 96, 571 Risk in “vaccine” group 7. 83/10, 000 Risk in “no vaccine” group 7. 96/10, 000 Relative Risk = 0. 98 Madsen et al. N Eng J Med 2002; 347: 1477 -82 25

Postlicensure Vaccine Safety Activities • Phase IV Trials - ~10, 000 participants - better but still limited • Large Linked Databases • Clinical Evaluation Network 26

Vaccine Safety Datalink • Large linked database • Links vaccination and health records • Population under “active surveillance” - 9 HMOs - >2. 5% (8 million) of U. S. population • Powerful tool for monitoring vaccine safety 27

• Improve understanding of vaccine safety • • • issues at individual level Evaluate individuals who experience adverse health events Gain better understanding of events Develop protocols for health care providers www. vaccinesafety. org/CISA 28

Vaccine Injury Compensation Program • Established by National Childhood Vaccine Injury Act (1986) • “No fault” program • Covers all routinely recommended childhood vaccines • Vaccine Injury Table • Reportable Events Table • www. hrsa. gov/vaccinecompensation 29

Parental Vaccine Safety Concerns in 2009 • >1 in 5 believe vaccines cause autism • 1 in 8 parents has refused at least 1 vaccine recommended by the clinician • 90% believe vaccines are good way to protect against diseases • 88% will do what the clinician recommends Freed et al, , J of Peds, March 2010 30

Personal Belief Exemptions • Permitting personal belief exemptions and easily granting exemptions are associated with higher and increasing nonmedical U. S. exemption rates • State policies granting personal belief exemptions and states that easily grant exemptions are associated with increased pertussis incidence JAMA. 2006; 296: 1757 -1763 31

Children With Personal Belief Exemption • 9 -fold higher risk of varicella (Colorado, 1998 -2008) • 23 -fold higher risk of pertussis (Colorado, 1996 -2007) • Introduce vaccine-preventable diseases (particularly measles) into school settings • Expose children with medical exemptions to infection 32

Reducing Vaccine Hesitancy and Personal Belief Exemptions • Engage the parent and answer their questions if possible • Be sure the parent understands that unvaccinated students will be excluded from school in the event of an outbreak • Provide the parent with information • Suggest reliable websites for further information (some are listed on IAC “What If” fact sheet) 33



Recent (and ongoing) Vaccine Safety Concerns by the Public • MMR and autism • Additives (particularly thimerosal) in vaccines • “Immune ovreload” • Too many shots 36




Autism and Vaccines • Multiple population-based studies have examined the rate of autism among vaccinated and unvaccinated children • Available evidence does not indicate that autism is more common among children who receive MMR or thimerosalcontaining vaccines than among children who do not receive vaccines 40

Studies of Autism and Vaccines* Taylor, B, et al. Autism and measles, mumps, and rubella vaccine: no epidemiologic evidence for a causal association. Lancet 351: 2026 -2029, 1999. Kaye JA, et al. Measles, mumps, and rubella vaccine and incidence of autism recorded by general practitioners: a time-trend analysis. Brit Med J 322: 460 -463, 2001. Madsen KM, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002; 347: 1477 -1482. Frambonne E, et al. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics 118: e 139 -50, 2006. Thompson WW, et al. Early thimerosal exposure and neuro-psychological outcomes at 7 to 10 years. N Engl J Med 2007; 357(13): 1281 -92. Schechter R, Grether JK. Continuing increases in autism reported to California's developmental services system: mercury in retrograde. Arch Gen Psychiatry 2008; 65(1): 19 -24. *partial listing of representative studies 41


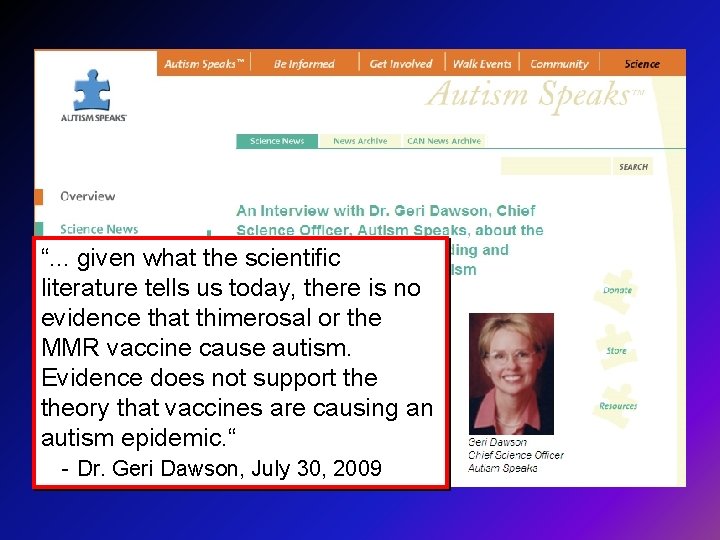
“. . . given what the scientific literature tells us today, there is no evidence that thimerosal or the MMR vaccine cause autism. Evidence does not support theory that vaccines are causing an autism epidemic. “ - Dr. Geri Dawson, July 30, 2009

Benefit and Risk Communication • Opportunities for questions should be provided before each vaccination • Vaccine Information Statements (VISs) - must be provided before each dose of vaccine - public and private providers - available in multiple languages 44

Your Source for VISs www. immunize. org

Providers Can Change Minds 2003 -2004 NIS interviews suggested: 1. 28% of parents doubtful about benefits & safety of certain vaccines 2. Doubtful parents delayed or refused their child's vaccination Most parents who changed their minds about delaying or refusing vaccination cited information from their physician as the main reason Oct 2008, J. of Pediatrics for the change 46

How to Have a Successful Dialogue with Parents • Take time to listen • Solicit and welcome questions • Keep the conversation going • Balance science with anecdotal information • Acknowledge benefits and risks 47

How to Have a Successful Dialogue with Parents • Respect parents’ authority • Reduce the stress of shots • Document parents’ questions and concerns • Follow up • Don’t give up 48

www. cdc. gov/vaccines/hcp/patient-ed/conversations/

www. cdc. gov/vaccines/hcp/patient-ed/conversations/




www. chop. edu/service/vaccine-education-center/home. html


Thank You
 Flat addressing vs hierarchical addressing
Flat addressing vs hierarchical addressing Sunday independent
Sunday independent William atkinson maryland
William atkinson maryland Overcoming trials and temptations
Overcoming trials and temptations A technique for overcoming internal fragmentation
A technique for overcoming internal fragmentation Overcoming the dark side of leadership
Overcoming the dark side of leadership Overcoming barriers to employment
Overcoming barriers to employment Scripture about overcoming adversity
Scripture about overcoming adversity What is communication barriers
What is communication barriers Hindrances to spiritual growth
Hindrances to spiritual growth All the blocks you want
All the blocks you want Roz shafran
Roz shafran Blessoff overcoming
Blessoff overcoming Writing a narrative about overcoming a challenge
Writing a narrative about overcoming a challenge Overcoming challenges essay
Overcoming challenges essay Overcoming glossophobia
Overcoming glossophobia Overcoming the monster examples
Overcoming the monster examples Information processing theory by atkinson and shiffrin
Information processing theory by atkinson and shiffrin Fuzzy traces
Fuzzy traces Atkinson and shiffrin model
Atkinson and shiffrin model Atkinson and shiffrin's three-stage model of memory
Atkinson and shiffrin's three-stage model of memory Atkinson shiffrin model of memory
Atkinson shiffrin model of memory Organizing items into familiar manageable units
Organizing items into familiar manageable units Ap psychology chapter 9 memory
Ap psychology chapter 9 memory Atkinson and shiffrin 1971
Atkinson and shiffrin 1971 Maudie atkinson
Maudie atkinson Who is jean louise in to kill a mockingbird
Who is jean louise in to kill a mockingbird To kill a mockingbird chapter 5
To kill a mockingbird chapter 5 Atkinson's flexible firm model
Atkinson's flexible firm model Dr wayne atkinson
Dr wayne atkinson Atkinson formula ventilation
Atkinson formula ventilation Benny st constant
Benny st constant Bisogni omeostatici
Bisogni omeostatici Atkinson
Atkinson Morphodite in to kill a mockingbird
Morphodite in to kill a mockingbird Miss maudie atkinson quotes chapters 4-6
Miss maudie atkinson quotes chapters 4-6 Scotland suicide statistics
Scotland suicide statistics Atkinson
Atkinson Lions of little rock by kristin levine
Lions of little rock by kristin levine Atkinson andelson loya ruud & romo
Atkinson andelson loya ruud & romo Motivazione atkinson
Motivazione atkinson Poppy atkinson
Poppy atkinson Dr christine atkinson
Dr christine atkinson Greer atkinson
Greer atkinson Answer the following question
Answer the following question What does miss maudie atkinson teach scout in chapters 4-6
What does miss maudie atkinson teach scout in chapters 4-6 Recencia hatás
Recencia hatás Vaccine storage and handling sop worksheet
Vaccine storage and handling sop worksheet Vaccine storage and handling protocol
Vaccine storage and handling protocol Addressing concerns and earning commitment
Addressing concerns and earning commitment Classful and classless addressing
Classful and classless addressing Difference between classful and classless addressing
Difference between classful and classless addressing Earning commitment
Earning commitment Difference between classful and classless addressing
Difference between classful and classless addressing Classless addressing example
Classless addressing example Addressing competition and driving growth ppt
Addressing competition and driving growth ppt Address cont example
Address cont example