Academic Chemistry Final Review Final Exam 51 Multiple













- Slides: 22

Academic Chemistry Final Review

Final Exam 51 Multiple Choice Periodic Trends – definition Atomic/Ionic Radius, Electronegativity, ionization energy, electron affinity Accuracy/Precision General Equations Valence Electrons Octet Types of Energy metals/nonmetals/metalloids Pure substances – elements/compounds Mixtures – homogeneous/heterogeneous

Final Exam 51 Multiple Choice Balanced Equations Stoichiometry Polarity – bond & molecule (s), (l), (g), (aq) VSEPR anion/cation empirical/molecular formulas Heisenburg/De. Broglie/Hund/ Temperature scales based on family names

Final Exam 51 Multiple Choice covalent/ionic Rutherford frequency/wavelength/amplitude Effect of changes on gases Gas pressure parts of the scientific method separation methods alpha/beta/gamma electrons on a sublevel indicators of a chemical change

Final Exam 51 Multiple Choice conservation of mass/energy significant figures physical/chemical changes periodic table arrangement limiting reactant molar mass atom/molecule/formula unit protons/neutrons/electrons Thompson

Final Exam 9 problems limiting reactant problem empirical and molecular formula write a balanced formula & determine type Differences between solids/liquids/gases % yield density atom/ion/isotope ideal gas law names/formulas

Books must be turned in before you leave! You may turn them in during class any day this week!

Review Chapters 1 -4 1. 68. 59 m = 4 s. f. 2. 0. 0003050 = 4 s. f 3. Steps 1. Identify the problem or question 2. Gather information and observations 3. Form a hypothesis 4. Test the hypothesis 5. Analyze the data and form a conclusion 4. hypothesis – educated guess about a problem theory – tested hypothesis law – tested many times, scientific community believes it to be true.

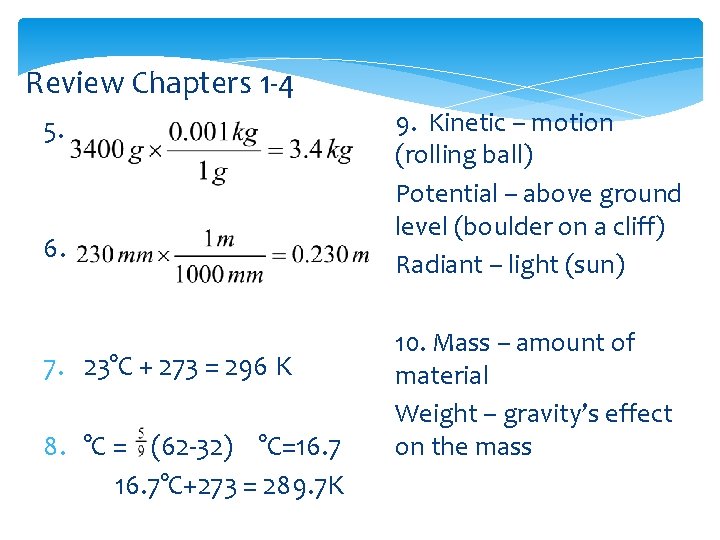
Review Chapters 1 -4 5. 6. 7. 23°C + 273 = 296 K 8. °C = (62 -32) °C=16. 7°C+273 = 289. 7 K 9. Kinetic – motion (rolling ball) Potential – above ground level (boulder on a cliff) Radiant – light (sun) 10. Mass – amount of material Weight – gravity’s effect on the mass

Review Chapters 1 -4 11. a. element – atom b. Covalent - molecule c. Ionic – Formula Unit 15. Aufbau Principle – Electrons are added one at a time to the lowest energy level available. Pauli Exclusion Principle – An orbital can hold a maximum 12. Mendeleev of 2 electrons. These electrons must have opposite 13. Protons = 92 spins (clockwise & counter. Neutrons (233 -92) = 141 clockwise). Electrons = 92 Hund’s Rule – Electrons fill orbitals so that a maximum 14. Mass of Carbon - 12 number of unpaired electrons exist. (Ladies first!)

Review Chapters 1 -4 16. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 105 p 66 s 24 f 145 d 9 17. [Xe]6 s 24 f 7 18. Ion – different # of electrons giving overall charge Isotope – different # of neutrons (different mass) 19. a. Dalton – Dalton’s atomic theory (from ideas of Democritus) b. Thomson – Cathode Ray Tube experiments c. Rutherford – Gold Foil Experiment 20. electron < proton = neutron < nucleus

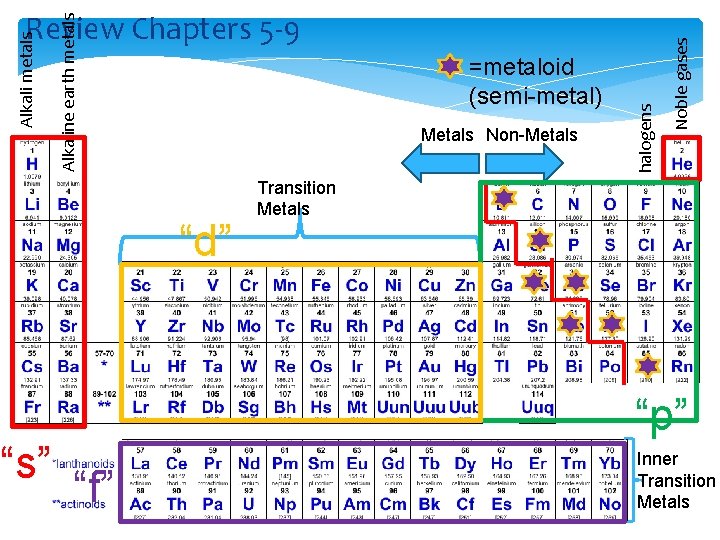
Metals Non-Metals 1. “d” “s” Noble gases =metaloid (semi-metal) halogens Alkaline earth metals Alkali metals Review Chapters 5 -9 Transition Metals “p” “f” Inner Transition Metals

Review Chapters 5 -9 2. a. Cl-1 d. Al+3 b. Na+1 e. N-3 c. Mg+2 f. S-2 3. An octet 4. Ionic – metal/non-metal Covalent – 2 non-metals 5. Valence Shell Electron Pair Repulsion Theory 6. Polar – unequal sharing of electrons – causing partial positive and partial negative determine by difference in electronegativity

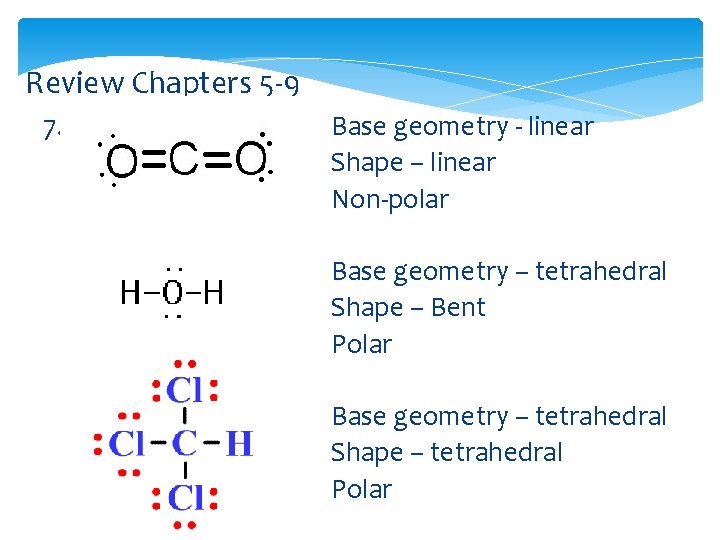
Review Chapters 5 -9 7. Base geometry - linear Shape – linear Non-polar Base geometry – tetrahedral Shape – Bent Polar Base geometry – tetrahedral Shape – tetrahedral Polar

Review Chapters 5 -9 8. a. sulfate b. ammonium 9. a. synthesis b. single replacement c. combustion c. phosphate d. carbonate d. decomposition e. double replacement 10. A molecule that exists in nature as two atoms bonded together. Br I N Cl H O F 11. light/heat. Precipitate. Gas. Color change.

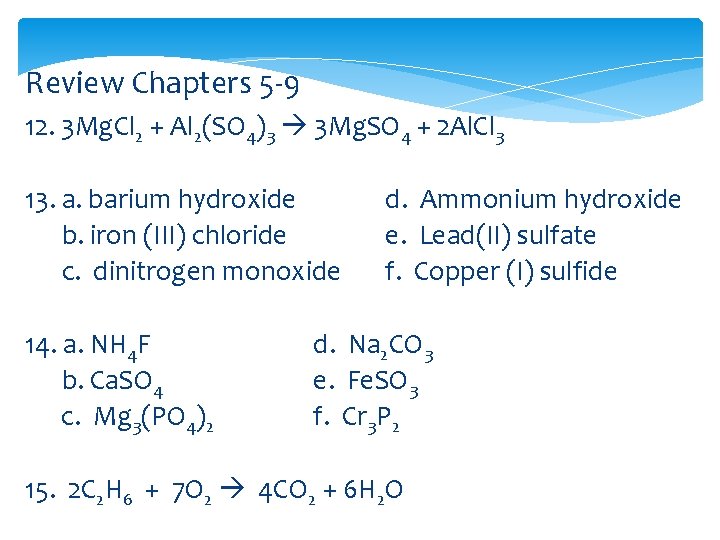
Review Chapters 5 -9 12. 3 Mg. Cl 2 + Al 2(SO 4)3 3 Mg. SO 4 + 2 Al. Cl 3 13. a. barium hydroxide b. iron (III) chloride c. dinitrogen monoxide 14. a. NH 4 F b. Ca. SO 4 c. Mg 3(PO 4)2 d. Ammonium hydroxide e. Lead(II) sulfate f. Copper (I) sulfide d. Na 2 CO 3 e. Fe. SO 3 f. Cr 3 P 2 15. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O

Review Chapters 10 -13 1. a. 40. 0 g b. 342 g c. 183. 4 g d. 149. 0 g 2. Empirical = lowest ratio Molecular = actual ratios 3. No, we cancel in criss cross to get lowest ratio 4. 6. 02 x 1023 - number of particles in a mole

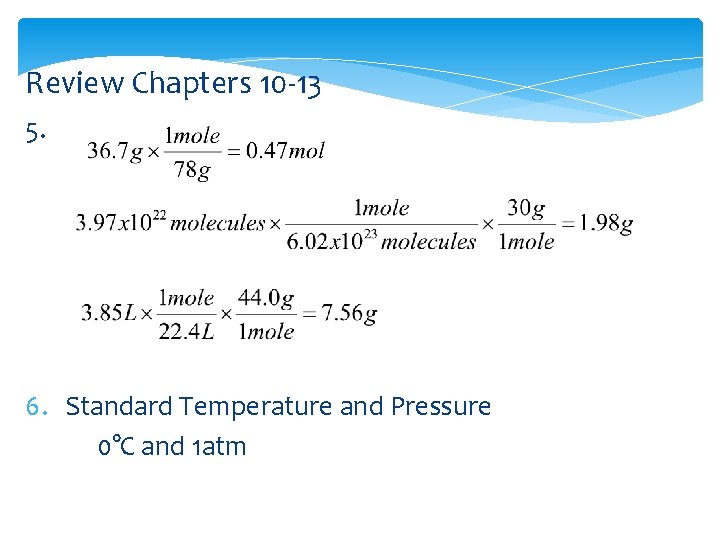
Review Chapters 10 -13 5. 6. Standard Temperature and Pressure 0°C and 1 atm

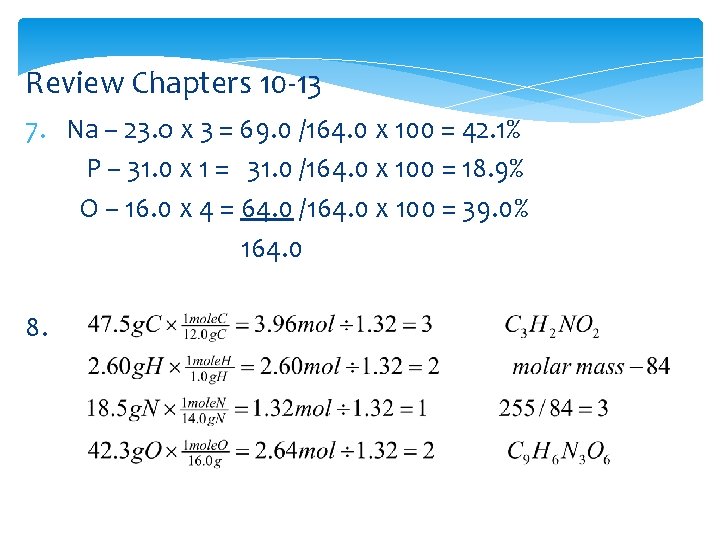
Review Chapters 10 -13 7. Na – 23. o x 3 = 69. 0 /164. 0 x 100 = 42. 1% P – 31. 0 x 1 = 31. 0 /164. 0 x 100 = 18. 9% O – 16. 0 x 4 = 64. 0 /164. 0 x 100 = 39. 0% 164. 0 8.

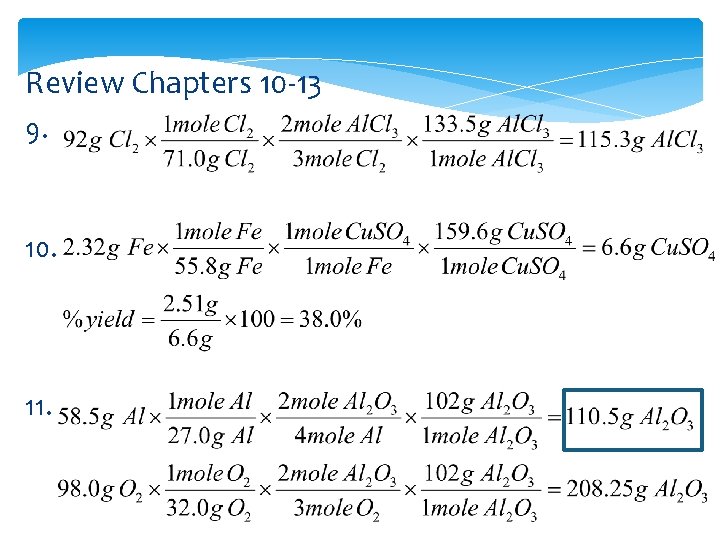
Review Chapters 10 -13 9. 10. 11.

Review Chapters 10 -13 12. Exo – releases heat Endo – absorbs heat 13. Enthalpy change – tells us how much heat is gained or lost during a reaction 14. The amount of heat needed to raise the temperature of 1 gram of a substance 1 degree C q= m. C∆T

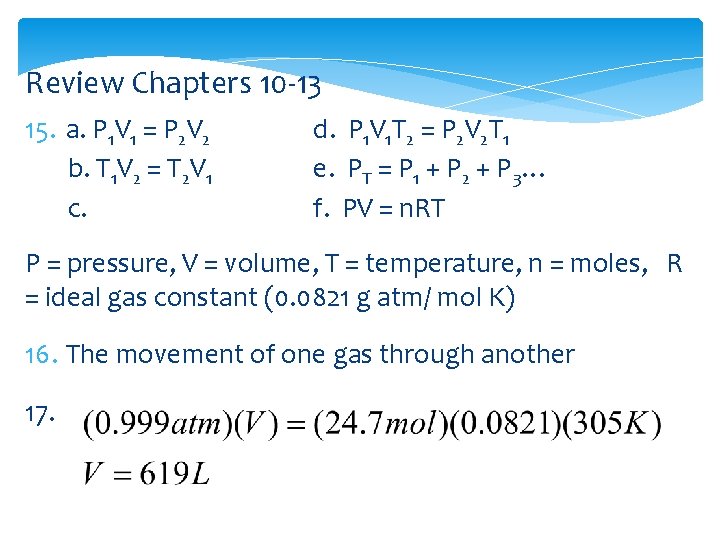
Review Chapters 10 -13 15. a. P 1 V 1 = P 2 V 2 b. T 1 V 2 = T 2 V 1 c. d. P 1 V 1 T 2 = P 2 V 2 T 1 e. PT = P 1 + P 2 + P 3… f. PV = n. RT P = pressure, V = volume, T = temperature, n = moles, R = ideal gas constant (0. 0821 g atm/ mol K) 16. The movement of one gas through another 17.