A Macroscopic Description of Matter Readings Chapter 16


- Slides: 23

A Macroscopic Description of Matter Readings: Chapter 16 1

Solid, Liquid, Gas -Solid has well-defined shape and well-defined surface. Solid is (nearly) incompressible. -Liquid has well-defined surface (not shape), It is (nearly) incompressible. - Gases are compressible. They occupy all volume. 2

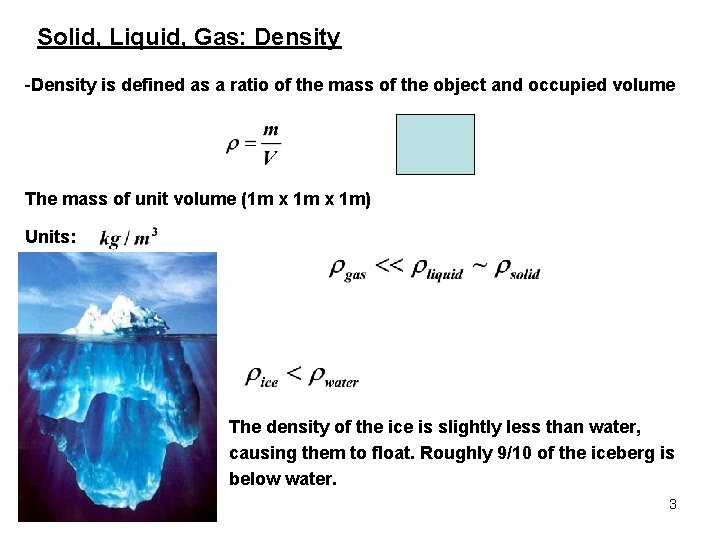
Solid, Liquid, Gas: Density -Density is defined as a ratio of the mass of the object and occupied volume The mass of unit volume (1 m x 1 m) Units: The density of the ice is slightly less than water, causing them to float. Roughly 9/10 of the iceberg is below water. 3

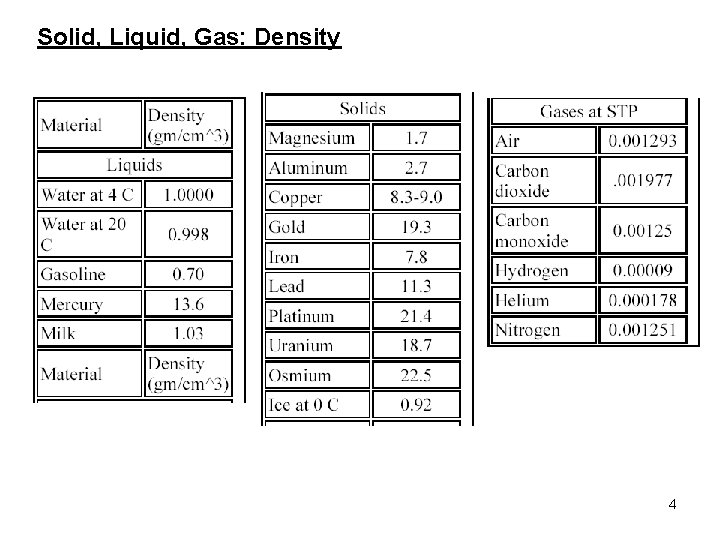
Solid, Liquid, Gas: Density 4

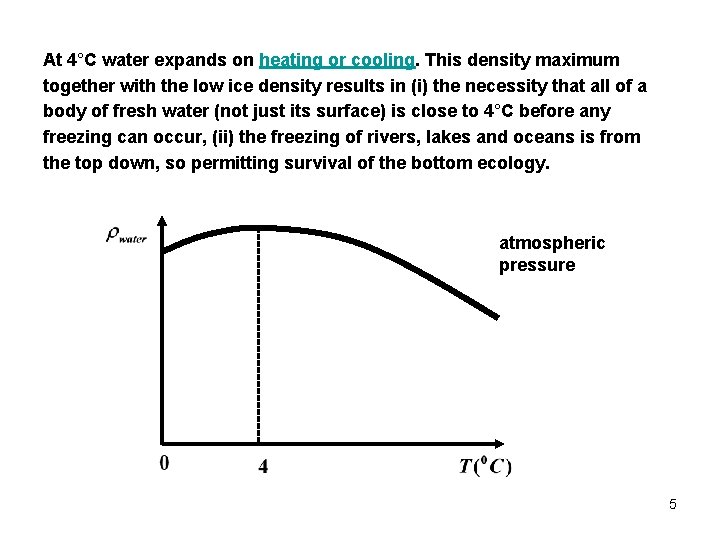
At 4°C water expands on heating or cooling. This density maximum together with the low ice density results in (i) the necessity that all of a body of fresh water (not just its surface) is close to 4°C before any freezing can occur, (ii) the freezing of rivers, lakes and oceans is from the top down, so permitting survival of the bottom ecology. atmospheric pressure 5

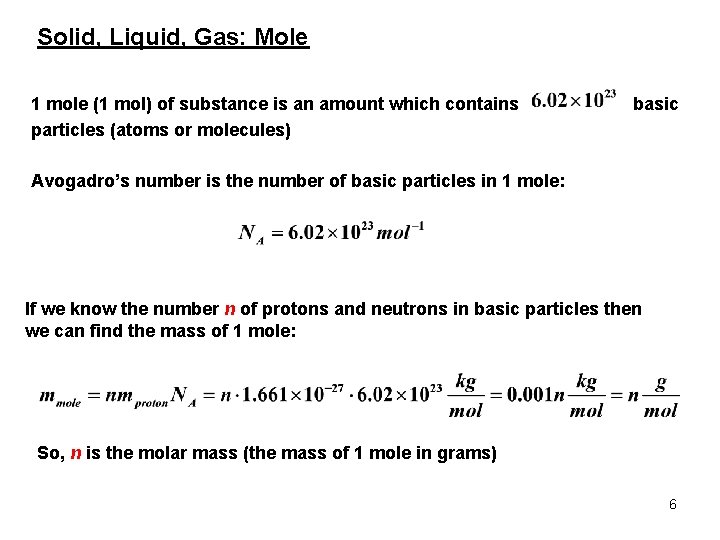
Solid, Liquid, Gas: Mole 1 mole (1 mol) of substance is an amount which contains particles (atoms or molecules) basic Avogadro’s number is the number of basic particles in 1 mole: If we know the number n of protons and neutrons in basic particles then we can find the mass of 1 mole: So, n is the molar mass (the mass of 1 mole in grams) 6

The number of protons and neutrons in atom 7

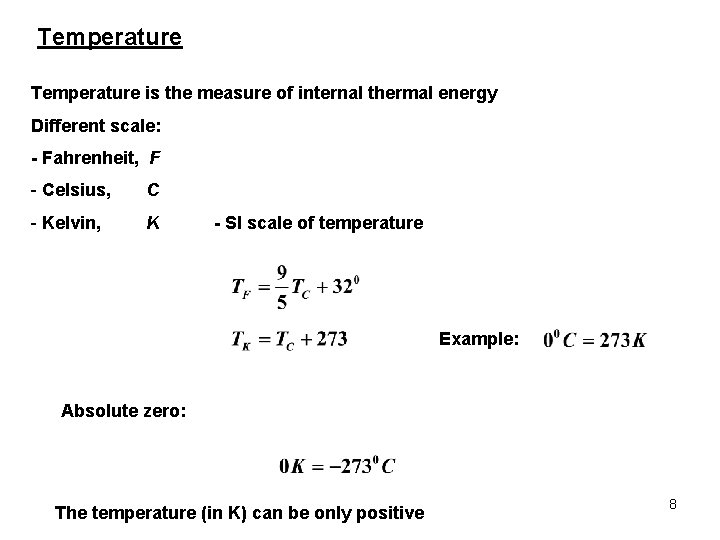
Temperature is the measure of internal thermal energy Different scale: - Fahrenheit, F - Celsius, C - Kelvin, K - SI scale of temperature Example: Absolute zero: The temperature (in K) can be only positive 8

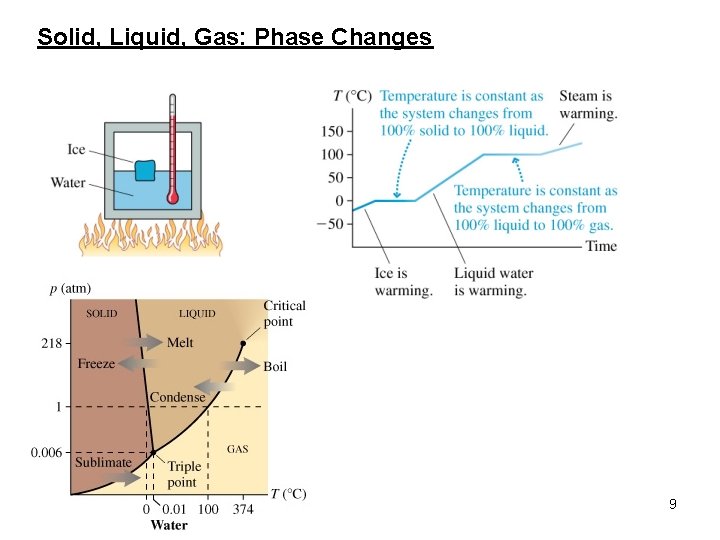
Solid, Liquid, Gas: Phase Changes 9

Heat: Specific Heat Specific heat of a substance is related to its thermal energy. Specific heat is defined as: The amount of energy that raises the temperature of 1 kg of a substance by 1 K is called specific heat, c. 10

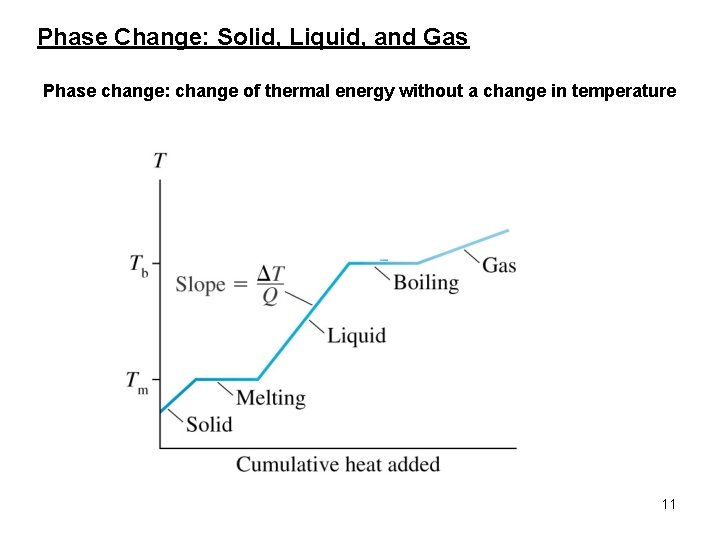
Phase Change: Solid, Liquid, and Gas Phase change: change of thermal energy without a change in temperature 11

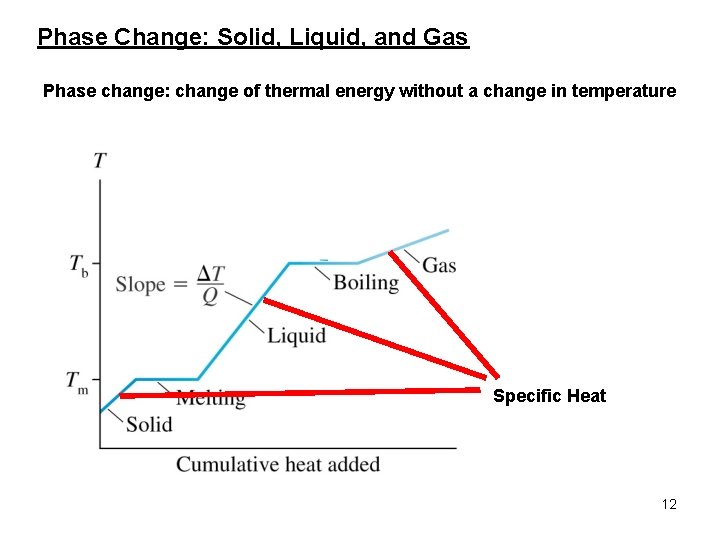
Phase Change: Solid, Liquid, and Gas Phase change: change of thermal energy without a change in temperature Specific Heat 12

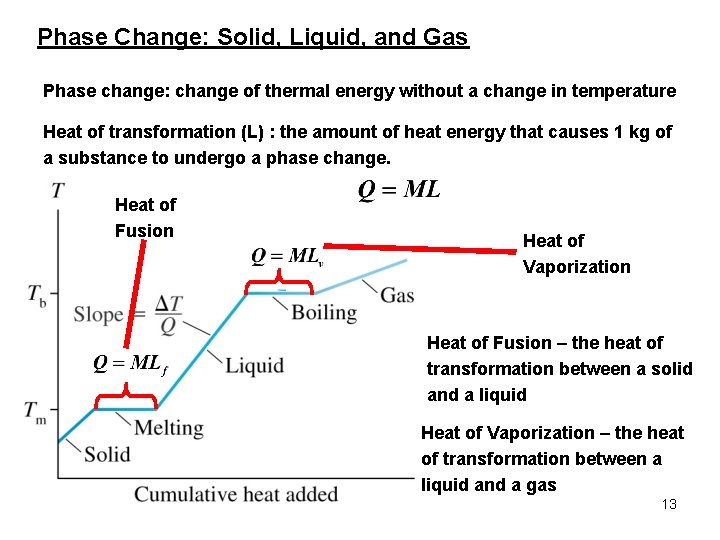
Phase Change: Solid, Liquid, and Gas Phase change: change of thermal energy without a change in temperature Heat of transformation (L) : the amount of heat energy that causes 1 kg of a substance to undergo a phase change. Heat of Fusion Heat of Vaporization Heat of Fusion – the heat of transformation between a solid and a liquid Heat of Vaporization – the heat of transformation between a liquid and a gas 13

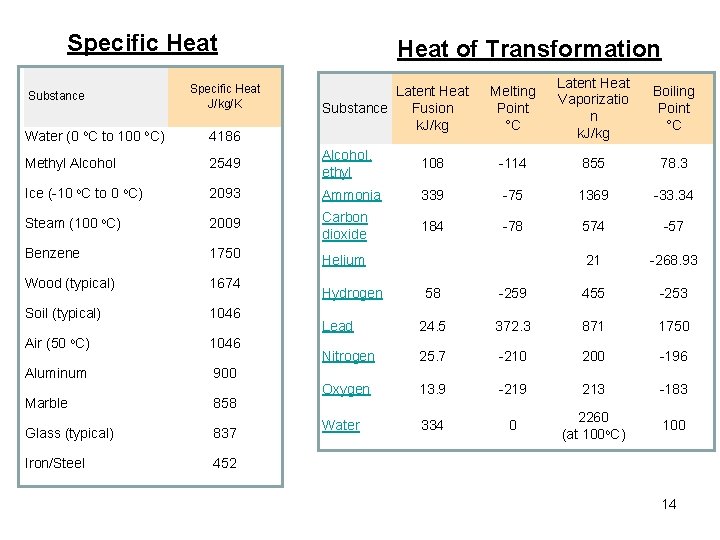
Specific Heat Substance Specific Heat J/kg/K Heat of Transformation Latent Heat Fusion Substance k. J/kg Melting Point °C Latent Heat Vaporizatio n k. J/kg Boiling Point °C Water (0 o. C to 100 o. C) 4186 Methyl Alcohol 2549 Alcohol, ethyl 108 -114 855 78. 3 Ice (-10 o. C to 0 o. C) 2093 Ammonia 339 -75 1369 -33. 34 Steam (100 o. C) 2009 Carbon dioxide 184 -78 574 -57 Benzene 1750 Helium 21 -268. 93 Wood (typical) 1674 Soil (typical) 1046 Air (50 o. C) 1046 Aluminum 900 Marble 858 Glass (typical) 837 Iron/Steel 452 Hydrogen 58 -259 455 -253 Lead 24. 5 372. 3 871 1750 Nitrogen 25. 7 -210 200 -196 Oxygen 13. 9 -219 213 -183 Water 334 0 2260 (at 100 o. C) 100 14

Ideal Gas 15

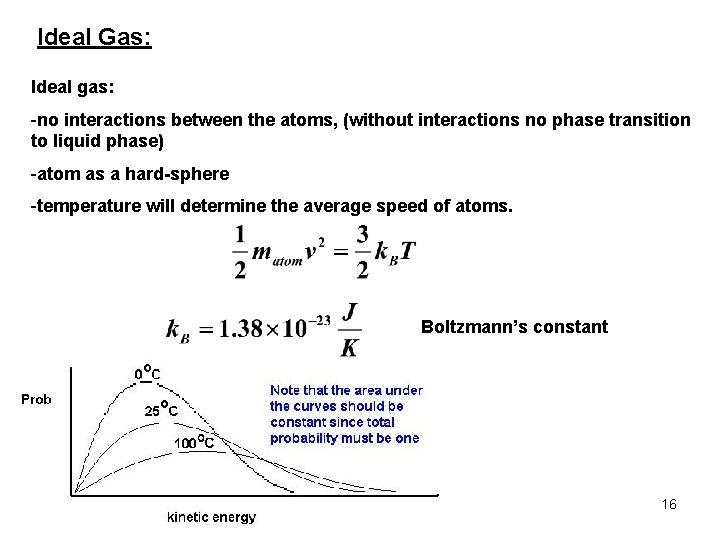
Ideal Gas: Ideal gas: -no interactions between the atoms, (without interactions no phase transition to liquid phase) -atom as a hard-sphere -temperature will determine the average speed of atoms. Boltzmann’s constant 16

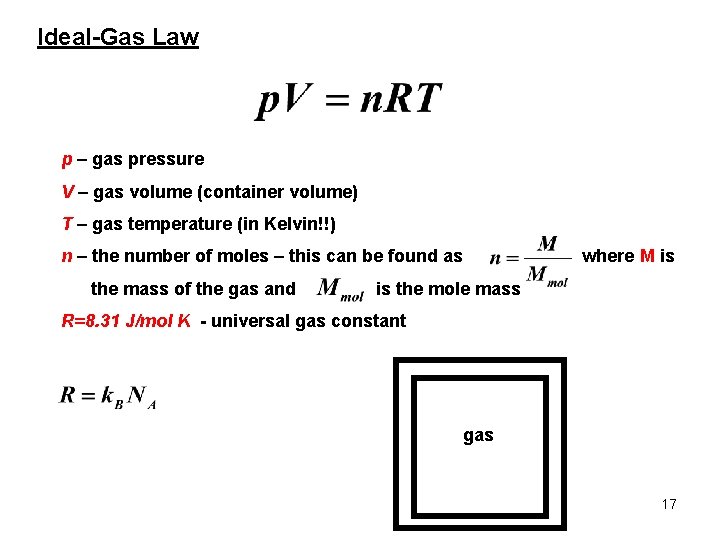
Ideal-Gas Law p – gas pressure V – gas volume (container volume) T – gas temperature (in Kelvin!!) n – the number of moles – this can be found as the mass of the gas and where M is is the mole mass R=8. 31 J/mol K - universal gas constant gas 17

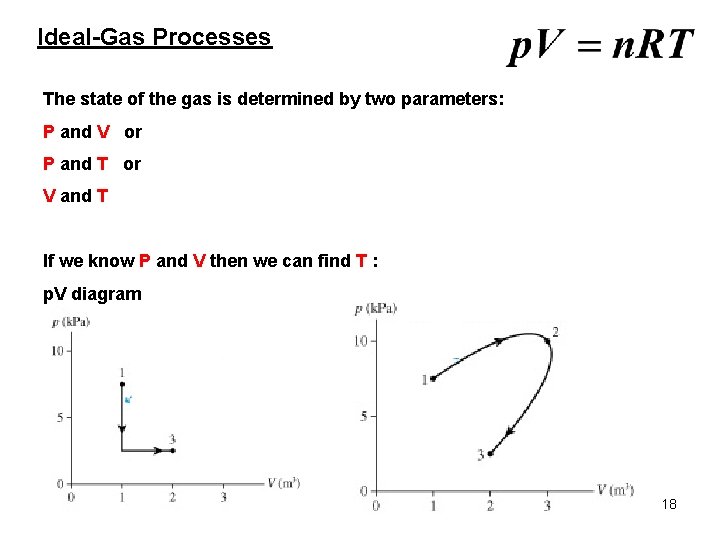
Ideal-Gas Processes The state of the gas is determined by two parameters: P and V or P and T or V and T If we know P and V then we can find T : p. V diagram 18

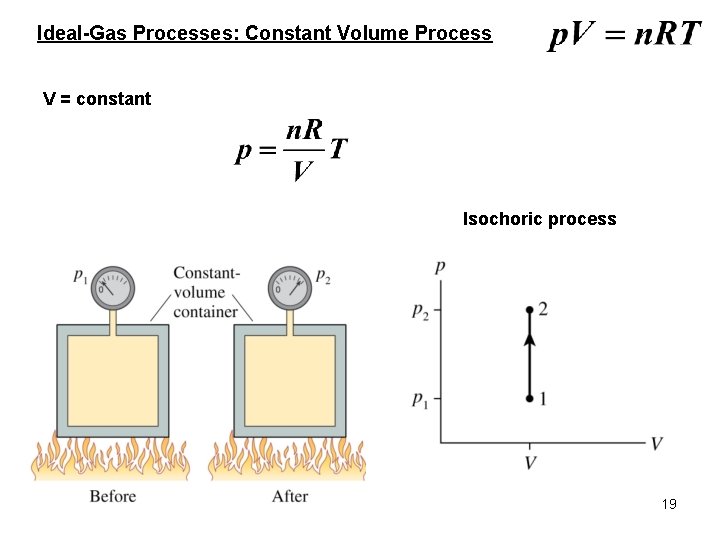
Ideal-Gas Processes: Constant Volume Process V = constant Isochoric process 19

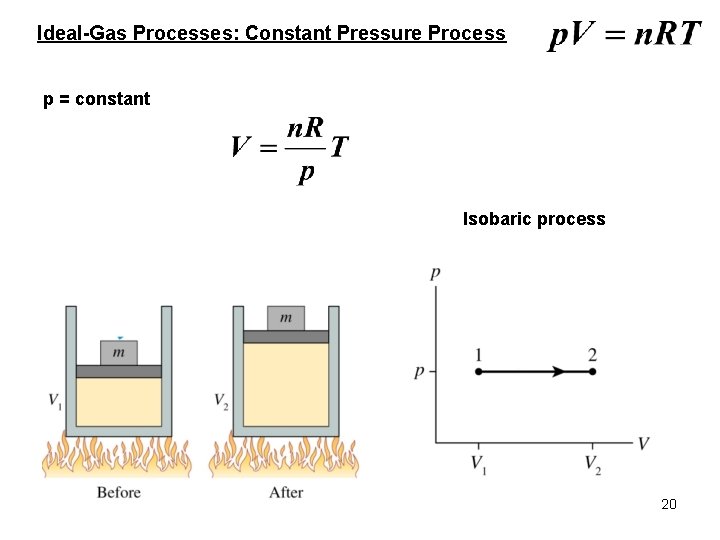
Ideal-Gas Processes: Constant Pressure Process p = constant Isobaric process 20

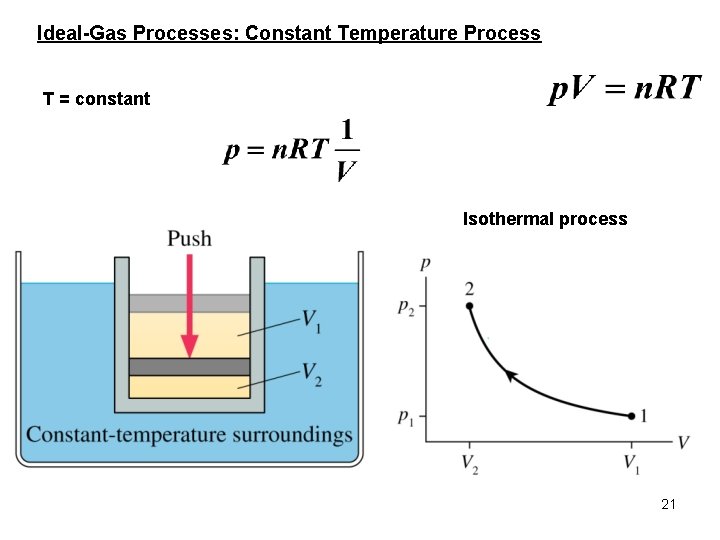
Ideal-Gas Processes: Constant Temperature Process T = constant Isothermal process 21

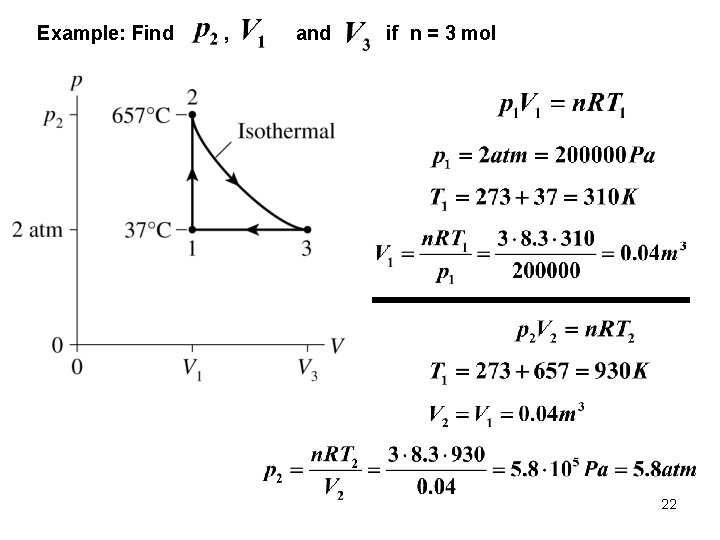
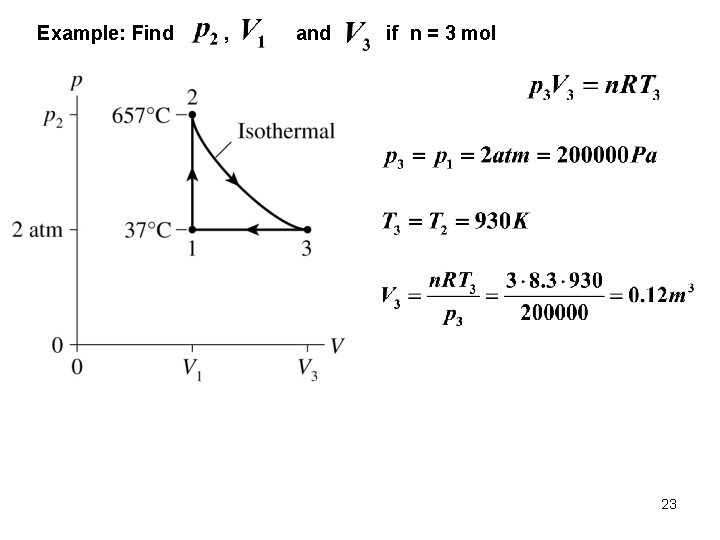
Example: Find , and if n = 3 mol 22

Example: Find , and if n = 3 mol 23