Chapter 1 Matter and Change Macroscopic View of


























- Slides: 43

Chapter 1: Matter and Change Macroscopic View of Matter • Matter that is large enough to be seen is called macroscopic, so all of your observations in chemistry, and everywhere else, start from this perspective. • You may get hints of the actual structure from a macroscopic view. You must go to a submicroscopic perspective to understand how the hidden structure of matter influences its behavior.

Submicroscopic View of Matter • The submicroscopic view gives you a glimpse into the world of atoms. • It is a world so small that you cannot see it even with the most powerful microscope, hence the term submicroscopic. • You learned in earlier science courses that matter is made up of atoms.

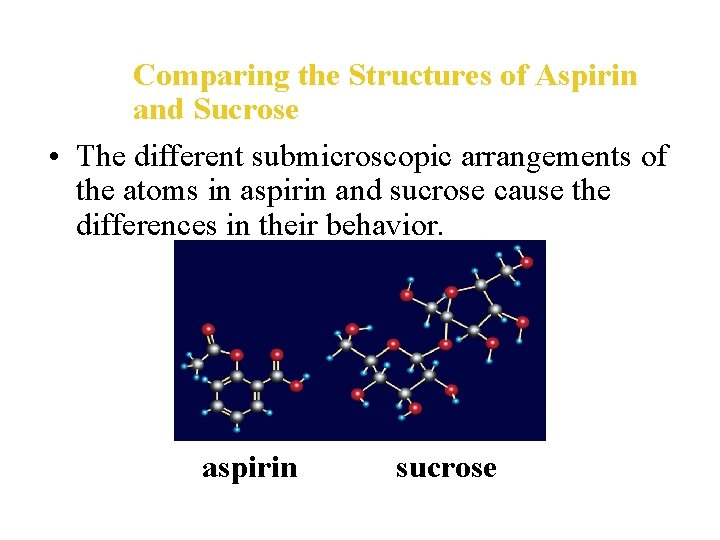
Using Models in Chemistry • In your study of chemistry, you will use both macroscopic and submicroscopic perspectives. • For example, sucrose and aspirin are both composed of carbon, hydrogen, and oxygen atoms, but they have different behaviors and functions. • These differences must come about because of differences in the submicroscopic arrangement of their atoms.

Comparing the Structures of Aspirin and Sucrose • The different submicroscopic arrangements of the atoms in aspirin and sucrose cause the differences in their behavior. aspirin sucrose

Amounts of Matter that Matter • Extenstive Properties – depend on the amount of matter present. (Mass, Volume and energy) • Intenstive Properties – are independent of the amount of matter present. (Boiling Point, Density and Temperature)

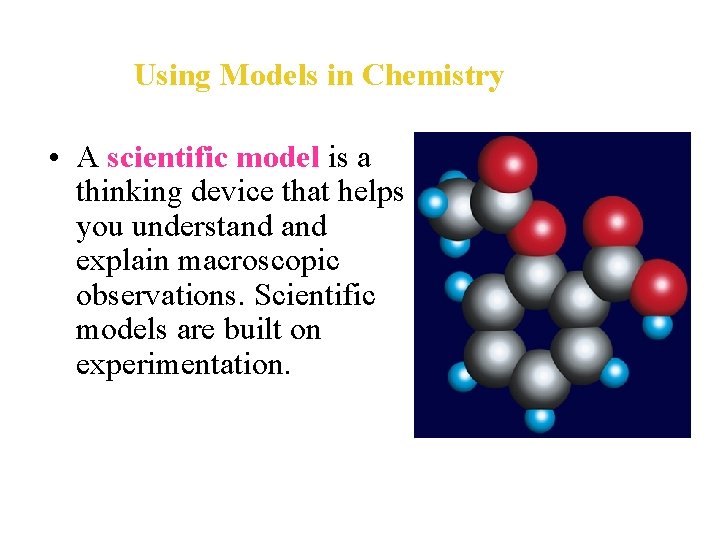
Using Models in Chemistry • A scientific model is a thinking device that helps you understand explain macroscopic observations. Scientific models are built on experimentation.

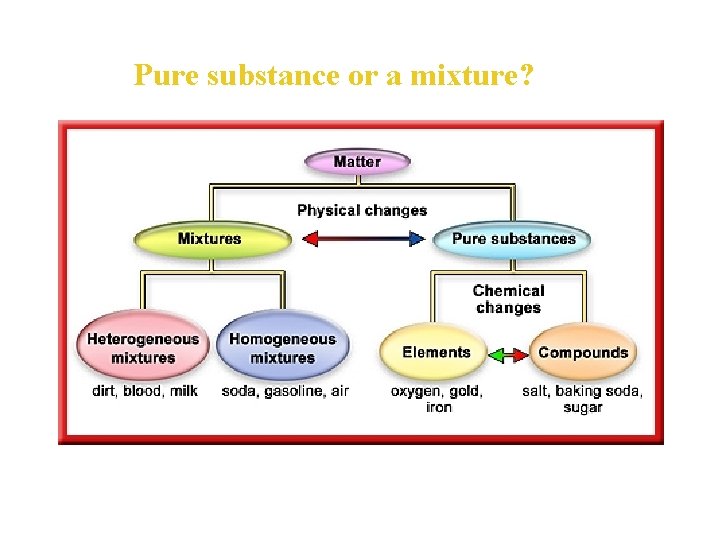
Pure substance or a mixture? • A sample of matter is either pure—made up of only one kind of matter— or it is a mixture of different kinds of matter. • A substance is matter, either an element or compound, with the same fixed composition and properties.

Pure substance or a mixture? • A mixture is a combination of two or more substances in which the basic identity of each substance is not changed. • Unlike pure substances, mixtures do not have specific compositions.

Pure substance or a mixture? • A physical change is a change in matter that does not involve a change in the chemical identity of individual substances. • Examples of physical changes include: • boiling, • evaporating, • freezing, • dissolving, • melting, • and crystallizing.

Pure substance or a mixture? • Physical properties are characteristics that a sample of matter exhibits without any change in its identity.

Pure substance or a mixture? • Examples of the physical properties of a chunk of matter include its: • solubility, • melting point, • boiling point, • color, • density, • electrical conductivity, • and physical state (solid, liquid, or gas).

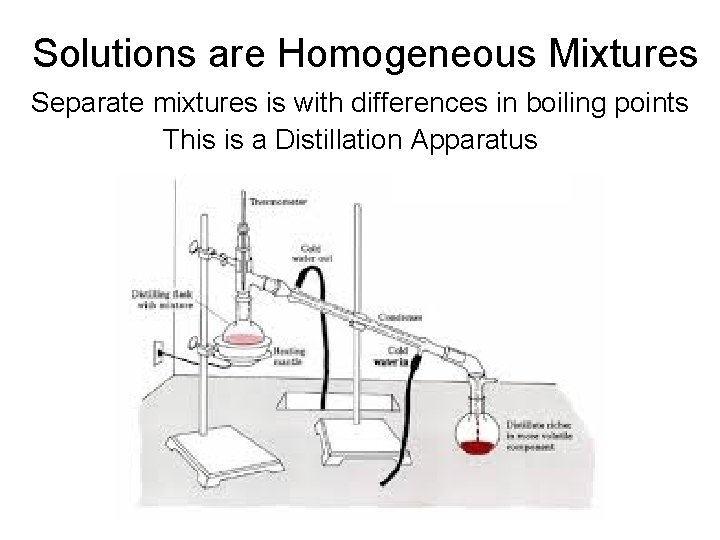
Solutions are Homogeneous Mixtures Separate mixtures is with differences in boiling points This is a Distillation Apparatus

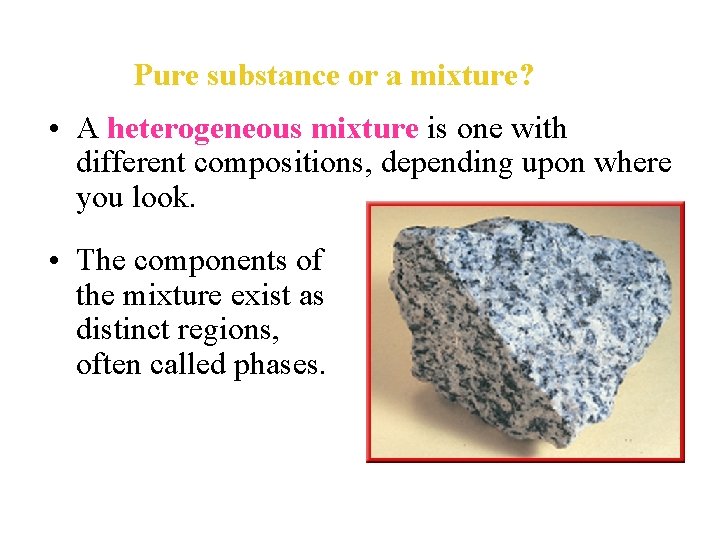
Pure substance or a mixture? • A heterogeneous mixture is one with different compositions, depending upon where you look. • The components of the mixture exist as distinct regions, often called phases.

Pure substance or a mixture? • Homogeneous mixtures are the same throughout. • Another name for a homogeneous mixture is solution. • Some solutions are gases. Air, for example, is a homogeneous mixture of several gases. • Some solutions are solid. • Liquid solutions do not have to be liquid or contain water.

Pure substance or a mixture? • Alloys are solid solutions that contain different metals and sometimes nonmetallic substances.

Pure substance or a mixture? • When you dissolve sugar in water, sugar is the solute—the substance being dissolved. • The substance that dissolves the solute, in this case water, is the solvent. • When the solvent is water, the solution is called an aqueous solution.

Pure substance or a mixture? • Many of the solutions you encounter are aqueous solutions, for example, soda, tea, contact-lens cleaner, and other cleaning liquids. • In addition, most of the processes of life occur in aqueous solutions.

Pure substance or a mixture?

Two Types of Substances • One type of pure substance can be broken down into simpler substances. This type of substance is called a compound. • Another type of substance cannot be broken down into simpler substances. Such a substance is called an element. • All the substances of the universe are either elements, compounds formed from elements, or mixtures of elements and compounds.

Two Types of Substances • Of the known elements, only about 90 occur naturally on Earth. The remainder are synthesized, usually in barely detectable amounts, in high-energy nuclear experiments. • Less than half of the 90 naturally occurring elements are abundant enough to play a significant role in the chemistry of everyday stuff.

Organizing the Elements • The periodic table organizes elements in a way that provides a wealth of chemical information—much more than is evident to you now. It shows the chemical symbols for the elements. • Their symbols usually correspond to their names in Latin.

Periodic Table of the Elements

Compounds Are More Than One Element • A more complete definition is that a compound is a chemical combination of two or more different elements joined together in a fixed proportion with a unique set of chemical and physical properties. • More than 10 million compounds are known and the number keeps growing.

Compounds Are More Than One Element • Some new compounds are discovered and isolated from natural chemical sources such as plants and colonies of bacteria and are synthesized in laboratories for many different uses.

Compounds Are More Than One Element • The properties of the compound are different from the properties of the elements that compose the compound. silver + bromine = silver bromide

Compounds Are More Than One Element • More than 10 million compounds are known and the number keeps growing. • New compounds are discovered and isolated from natural chemical sources such as plants and colonies of bacteria and are synthesized in laboratories for many different uses.

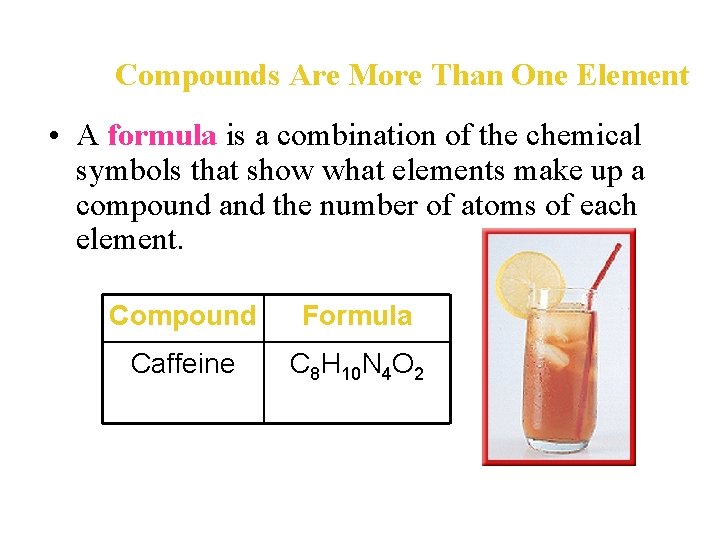
Compounds Are More Than One Element • A formula is a combination of the chemical symbols that show what elements make up a compound and the number of atoms of each element. Compound Formula Caffeine C 8 H 10 N 4 O 2

Compounds Are More Than One Element • Formulas provide a shorthand way of describing a submicroscopic view of a compound. • You probably already use formulas like H 2 O and CO 2 as a way of talking about water and carbon dioxide.

States of Matter • Most matter on Earth exists in one of three physical states: solid, liquid, or gas. A fourth state of matter, called plasma, is less familiar. • Changes in state are examples of physical changes because there is no change in the chemical composition identity of the substance. • Ice can melt back to form liquid water, and steam will condense on a cool surface to form liquid water.

States of Matter • Some substances are described as volatile, which means that they change to a gas easily at room temperature. • Alcohol and gasoline are more volatile than water. • Density is the amount of matter (mass) contained in a unit of volume. • Styrofoam has a low density or small mass per unit of volume.

States of Matter • Stones have a large density or a large mass per unit of volume. • In science, the density of solids and liquids is usually measured in units of grams (mass) per milliliter (volume) or g/m. L.

Chemical Properties • Chemical properties are those that can be observed only when there is a change in the composition of the substance. • Rusting is a chemical reaction in which iron combines with oxygen to form a new substance, iron oxide. • Inability to react is also a chemical property.

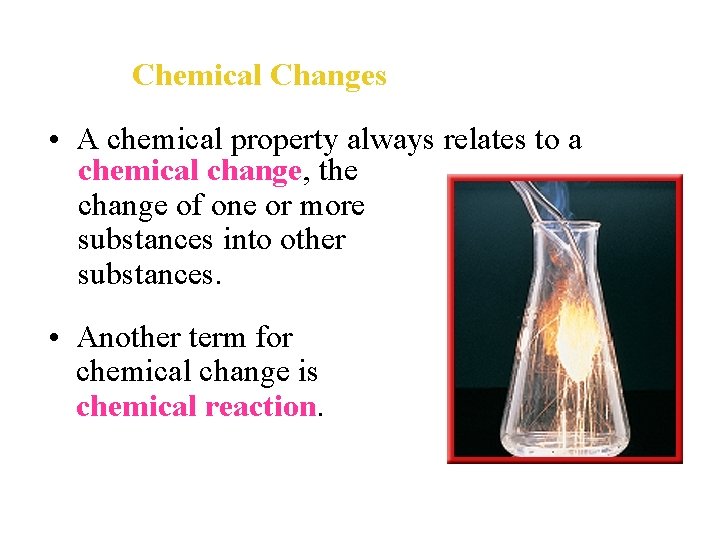
Chemical Changes • A chemical property always relates to a chemical change, the change of one or more substances into other substances. • Another term for chemical change is chemical reaction.

Chemical Changes • All matter is made of atoms, and any chemical change involves only a rearrangement of the atoms. Atoms do not just appear. Atoms do not just disappear. • This is an example of the law of conservation of mass, which says that in a chemical change, matter is neither created nor destroyed. It would be equally correct to call this the law of conservation of matter.

Chemical Reactions and Energy • All chemical changes also involve some sort of energy change. • Energy is either taken in or given off as the chemical change takes place. Energy is the capacity to do work. • Work is done whenever something is moved.

Chemical Reactions and Energy • Many reactions give off energy. • For example, burning wood is a chemical change in which cellulose, and other substances in the wood, combine with oxygen from the air to produce mainly carbon dioxide and water.

Chemical Reactions and Energy • Energy is also produced and released in the form of heat and light. • Chemical reactions that give off heat energy are called exothermic reactions. • Chemical reactions that absorb heat energy are called endothermic reactions.

Chemical Reactions and Energy • You can tell that the decomposition of water into oxygen and hydrogen is an endothermic reaction because it doesn’t occur unless energy, in the form of an electric current, is passed through water.

Chemical Reactions and Energy • Photosynthesis is probably the most important endothermic process on Earth. • Green plants, algae, and many kinds of bacteria carry out photosynthesis.

Assessment Questions Question 1 Identify each of the following as either a compound or a mixture. A. sand B. water C. juice

Assessment Questions Answers A. sand mixture B. water compound C. juice mixture

Assessment Questions Question 2 Classify each of the following as a chemical or physical property. A. density B. reactivity C. color D. melting point

Assessment Questions Answers A. density physical property B. reactivity chemical property C. color physical property D. melting point physical property