8 th Grade SOL LastMinute Review What hypothesis

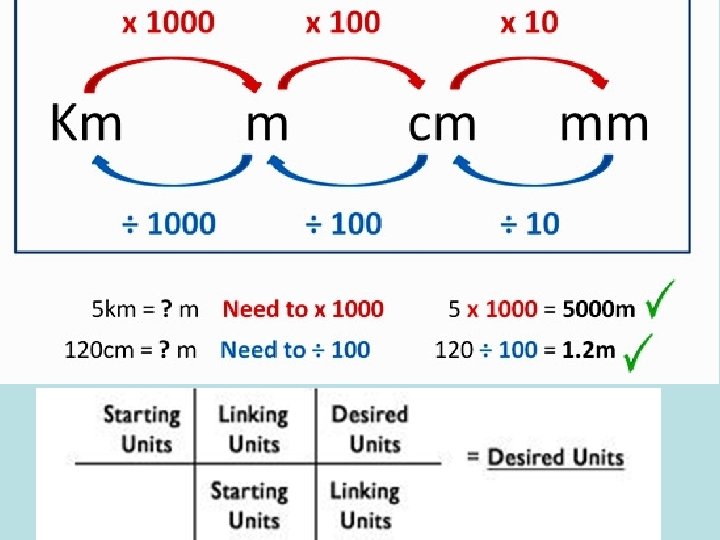













- Slides: 40

8 th Grade SOL Last-Minute Review


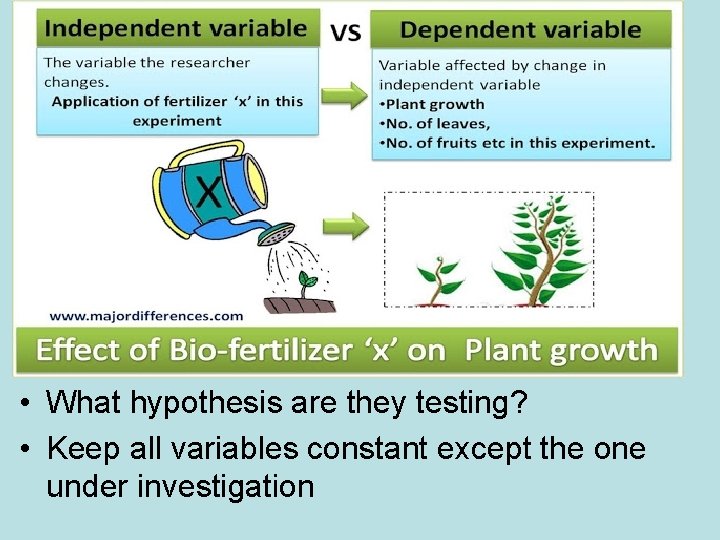
• What hypothesis are they testing? • Keep all variables constant except the one under investigation

The Nature of Science • Observation—data gathered by the senses • Inference—interpretation based on prior knowledge • Hypothesis—testable prediction of a relationship between 2 variables • Control group—left natural to be used as comparison

When looking at questions about a scientific experiment: • Decide what they are manipulating (independent variable!) • Decide what variable will respond and be the data they will collect (dependent variable!) • More trials =more trustworthy • Many times the answer is IN the question!!! • ONLY TEST one variable at a time

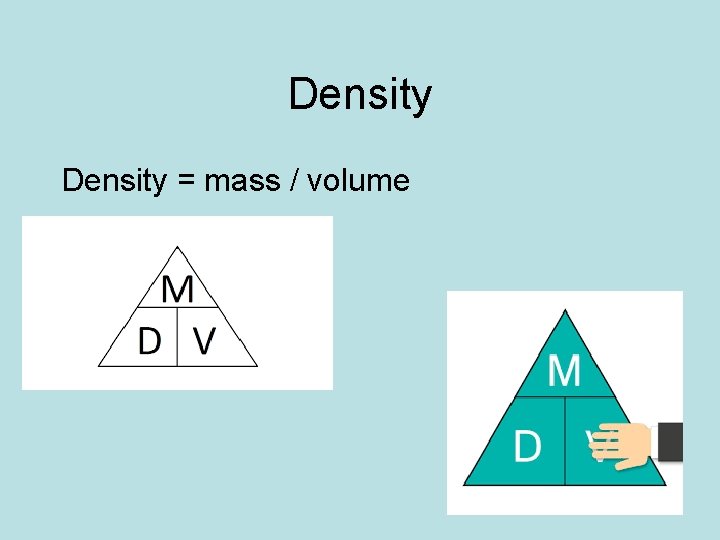
Density = mass / volume

Density of Water Density is a physical property The density of water is 1. 0 g/cm 3 An object or substance will float if its density is lower than water. An object or substance will sink if its density is higher than water. Oil floats on top of water because its density is lower than water.

Physical Properties Can be observed without changing the substance into another Physical Properties Include: Particle size Density (mass/volume) Melting or boiling points Hardness Thermal conductivity Electrical Conductivity Malleability or ductility

Chemical Properties include: Reactivity: does it undergo chemical reactions easily? will it rust? Flammability: the ability of a substance to burn

Chemical vs Physical changes and properties Substances can be classified by their physical properties. Metals are strong thermal and electrical Conductors. • Can it be observed without changing the substance? Magnetism, boiling etc… • Signs of chemical change: products formed (gas, precipitate, odors, colors) • Can be summarized by chemical equation (reactants react to form products) • Physical changes: changing size, changing state of matter

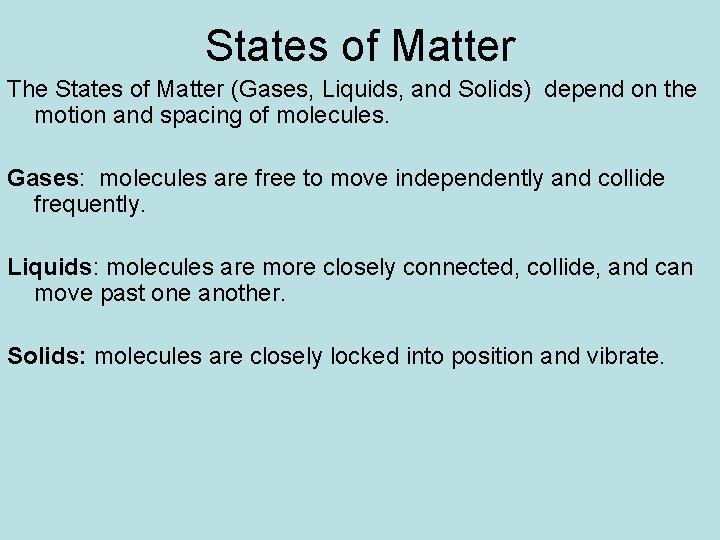
States of Matter The States of Matter (Gases, Liquids, and Solids) depend on the motion and spacing of molecules. Gases: molecules are free to move independently and collide frequently. Liquids: molecules are more closely connected, collide, and can move past one another. Solids: molecules are closely locked into position and vibrate.

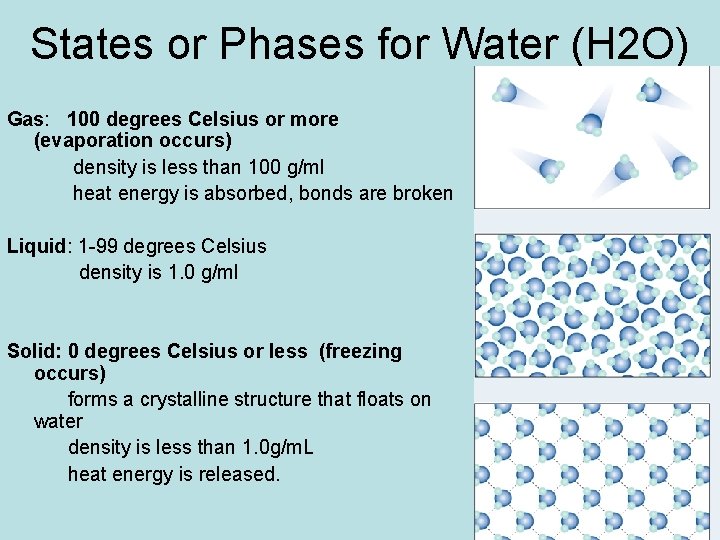
States or Phases for Water (H 2 O) Gas: 100 degrees Celsius or more (evaporation occurs) density is less than 100 g/ml heat energy is absorbed, bonds are broken Liquid: 1 -99 degrees Celsius density is 1. 0 g/ml Solid: 0 degrees Celsius or less (freezing occurs) forms a crystalline structure that floats on water density is less than 1. 0 g/m. L heat energy is released.

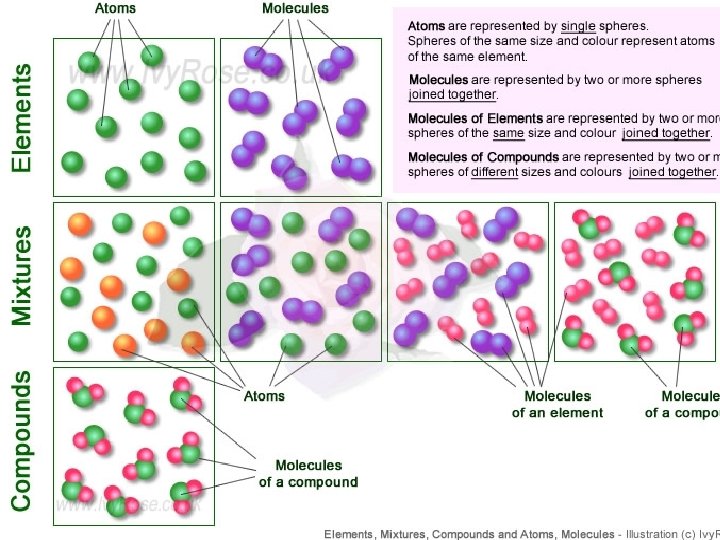
Each of more than the 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements.

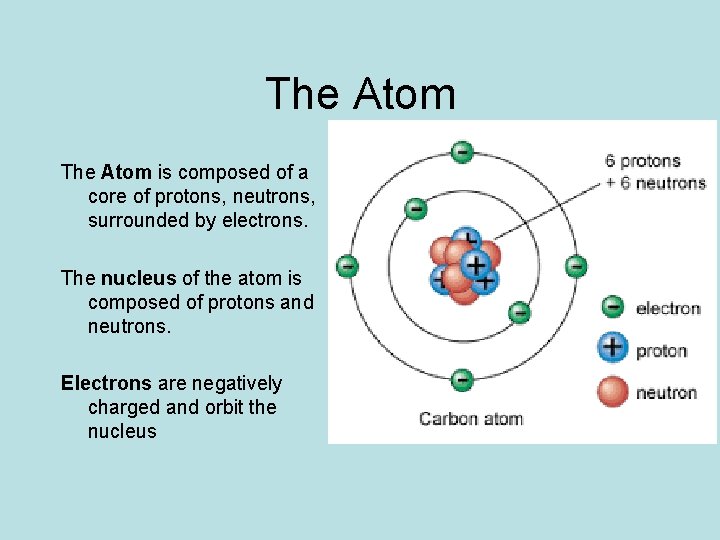
The Atom is composed of a core of protons, neutrons, surrounded by electrons. The nucleus of the atom is composed of protons and neutrons. Electrons are negatively charged and orbit the nucleus

Compounds are formed by combining 2 or more different elements together. Compounds have properties that are different from the individual elements. Example: H 20 water

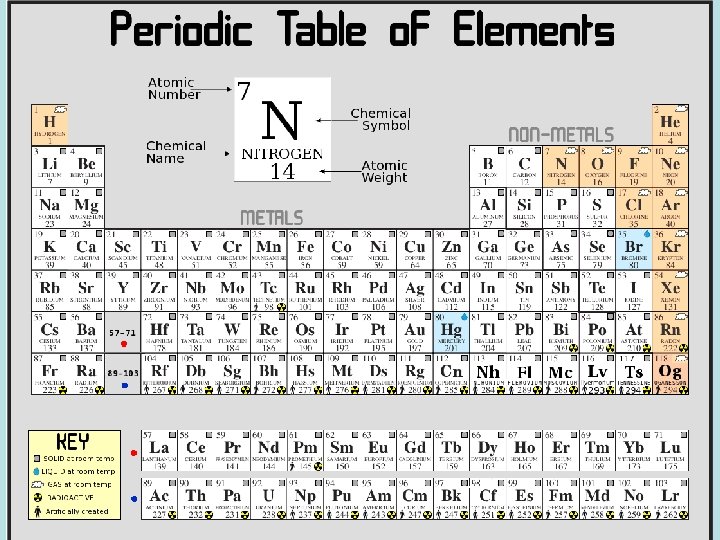
The Periodic table Periods: Each horizontal row in the chart. Changes in chemical properties of elements across the period correspond to changes in the electron arrangements of the atoms of the element. Groups: Each vertical column in the chart. The elements in the group have a similar chemical properties because their atoms have a similar number of electrons in their outer energy level. Main group number tells valence electron #

The Periodic Chart Be able to find: metals, nonmetals on the chart

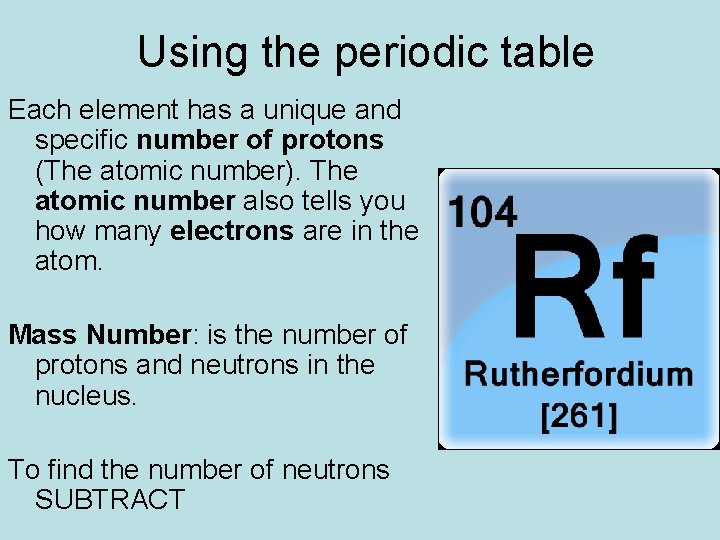
Using the periodic table Each element has a unique and specific number of protons (The atomic number). The atomic number also tells you how many electrons are in the atom. Mass Number: is the number of protons and neutrons in the nucleus. To find the number of neutrons SUBTRACT

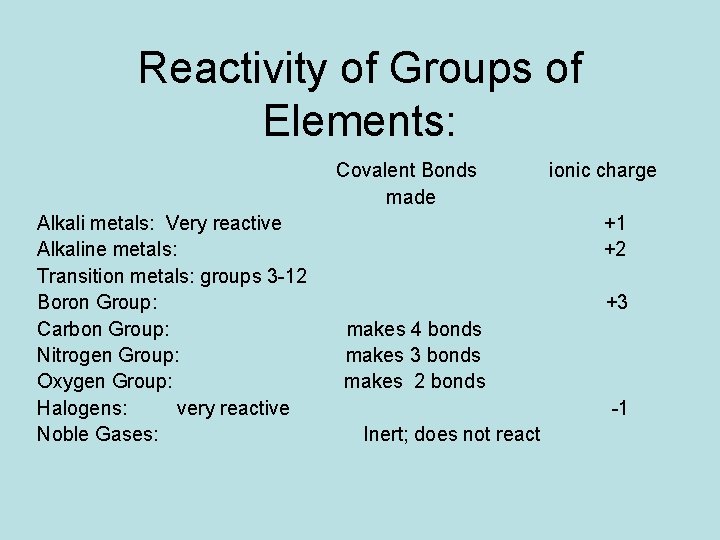
Reactivity of Groups of Elements: Covalent Bonds made Alkali metals: Very reactive Alkaline metals: Transition metals: groups 3 -12 Boron Group: Carbon Group: Nitrogen Group: Oxygen Group: Halogens: very reactive Noble Gases: ionic charge +1 +2 +3 makes 4 bonds makes 3 bonds makes 2 bonds -1 Inert; does not react

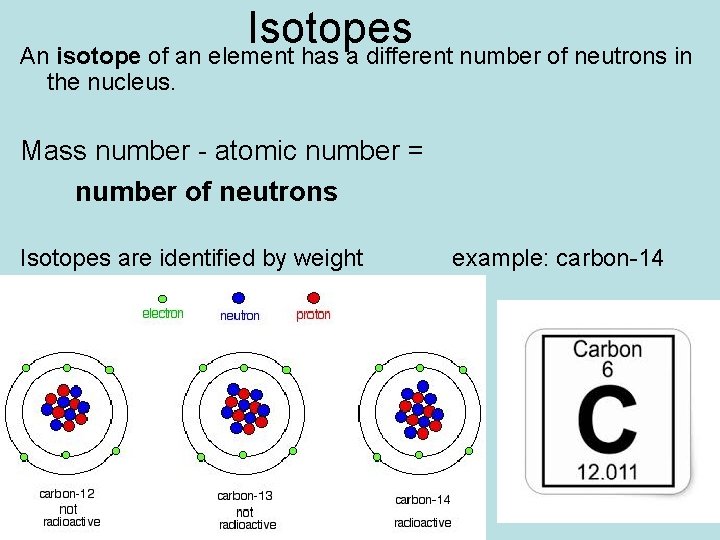
Isotopes An isotope of an element has a different number of neutrons in the nucleus. Mass number - atomic number = number of neutrons Isotopes are identified by weight example: carbon-14



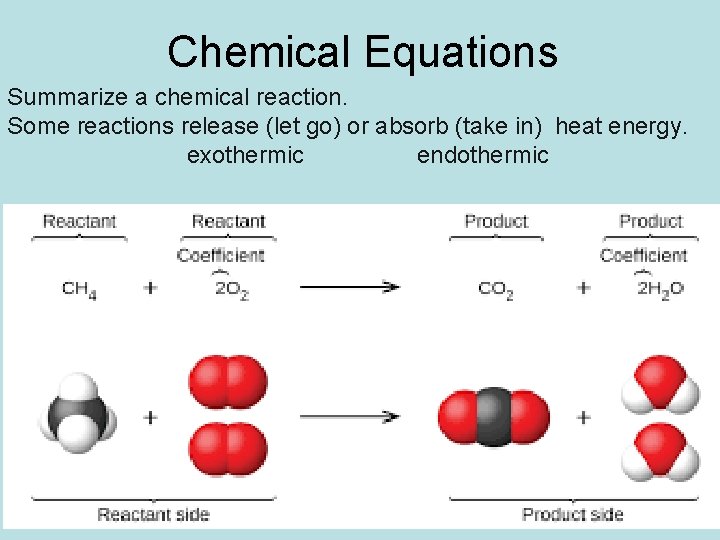
Chemical Equations Summarize a chemical reaction. Some reactions release (let go) or absorb (take in) heat energy. exothermic endothermic.

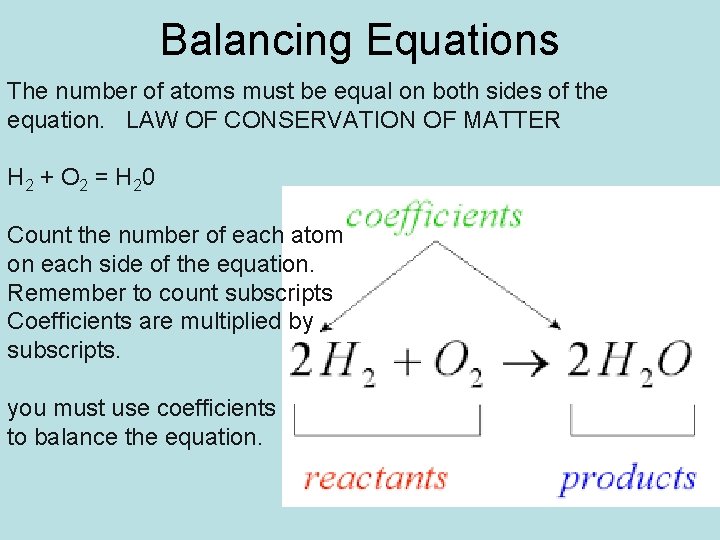
Balancing Equations The number of atoms must be equal on both sides of the equation. LAW OF CONSERVATION OF MATTER H 2 + O 2 = H 20 Count the number of each atom on each side of the equation. Remember to count subscripts Coefficients are multiplied by subscripts. you must use coefficients to balance the equation.


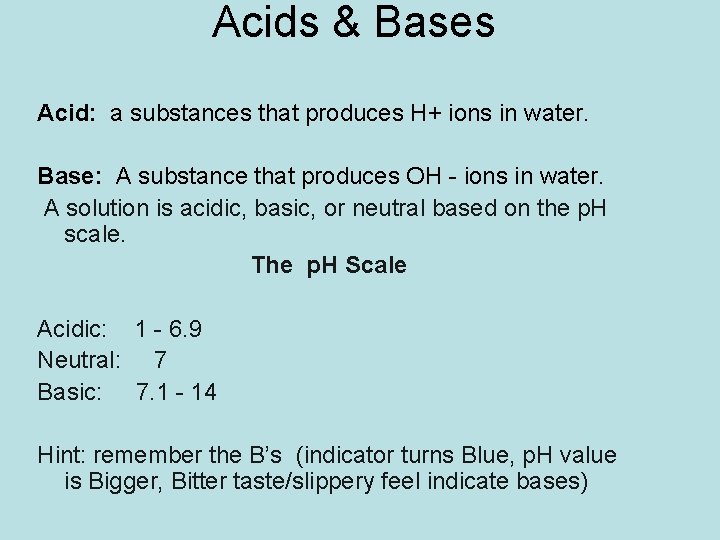
Acids & Bases Acid: a substances that produces H+ ions in water. Base: A substance that produces OH - ions in water. A solution is acidic, basic, or neutral based on the p. H scale. The p. H Scale Acidic: 1 - 6. 9 Neutral: 7 Basic: 7. 1 - 14 Hint: remember the B’s (indicator turns Blue, p. H value is Bigger, Bitter taste/slippery feel indicate bases)

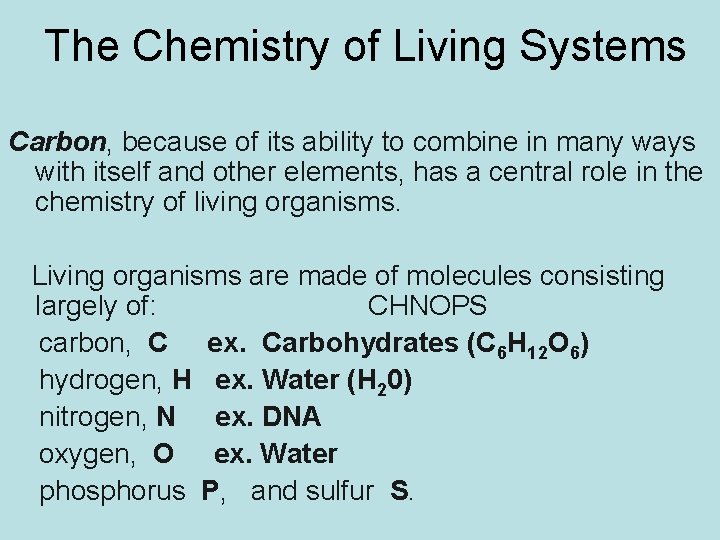
The Chemistry of Living Systems Carbon, because of its ability to combine in many ways with itself and other elements, has a central role in the chemistry of living organisms. Living organisms are made of molecules consisting largely of: CHNOPS carbon, C ex. Carbohydrates (C 6 H 12 O 6) hydrogen, H ex. Water (H 20) nitrogen, N ex. DNA oxygen, O ex. Water phosphorus P, and sulfur S.


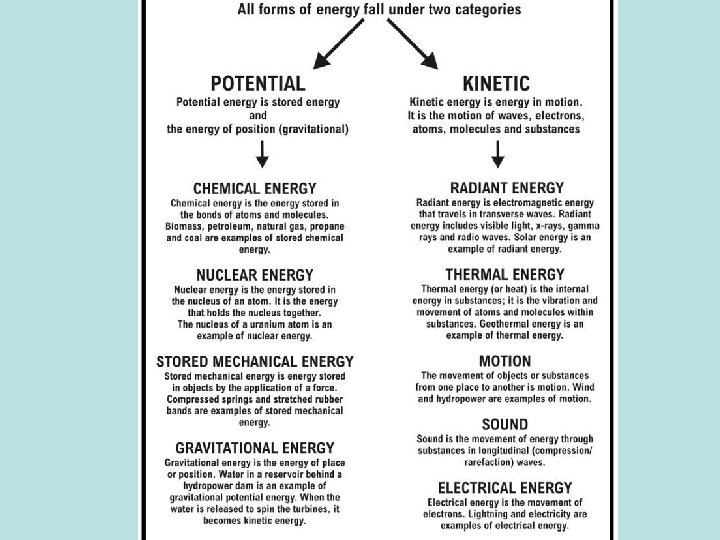
energy • Combustion reactions release carbon dioxide— a greenhouse gas. -- This is why we are concerned about the burning of non-renewable fossil fuels • Nuclear energy is non-renewable because we could run out of the right uranium isotopes. WASTE is the big issue with it • Renewable energy sources like wind, geothermal, waves, water, and solar are much better for our climate and health!

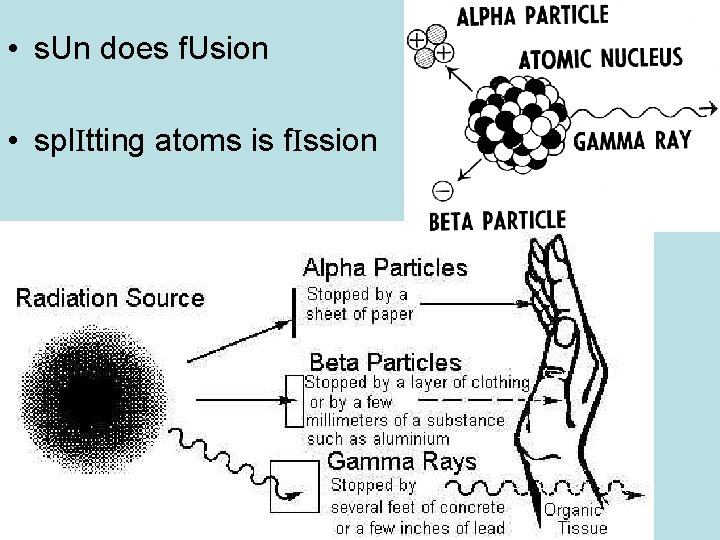
• s. Un does f. Usion • spl. Itting atoms is f. Ission



Gravity Acceleration due to gravity 9. 8 m/s 2 It gets stronger as mass increases and gets weaker as distance increases Weight is a force due to the mass of an object and the acceleration due to gravity acting upon it. Weight = Mass x 9. 8 m/s 2

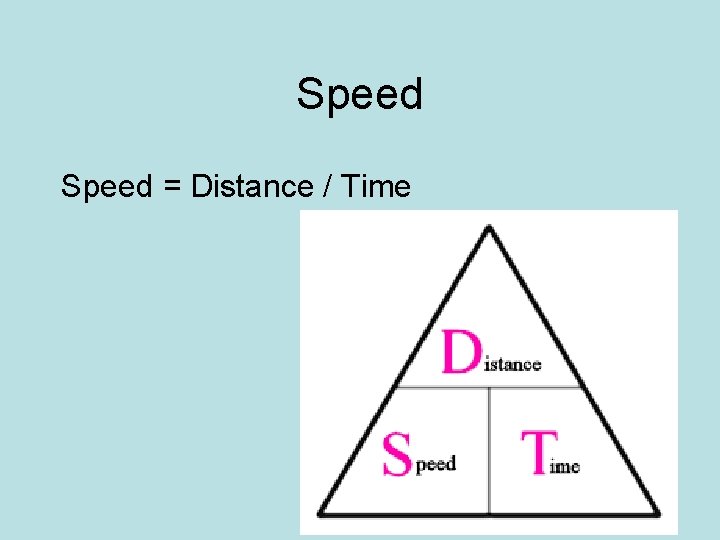
Speed = Distance / Time

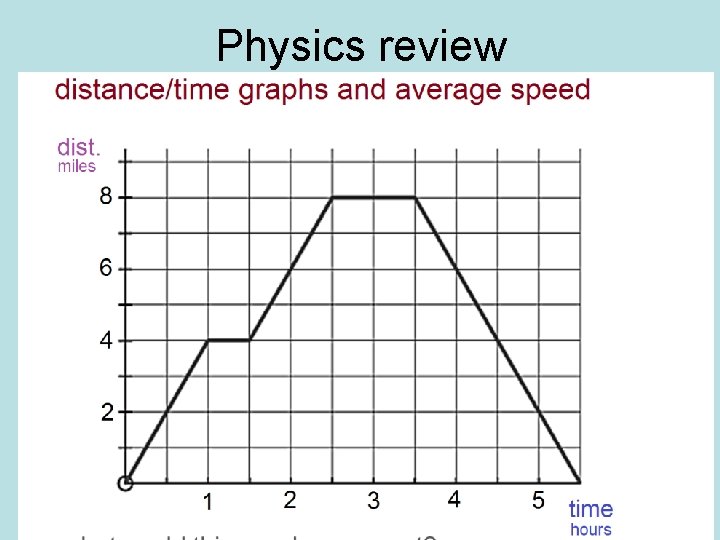
Physics review

Velocity is the rate of change in the position of an object. Velocity specifies both the speed and the direction of an object. (i. e. 40 m/s north) Velocity may be due to changes in speed, direction, or both.

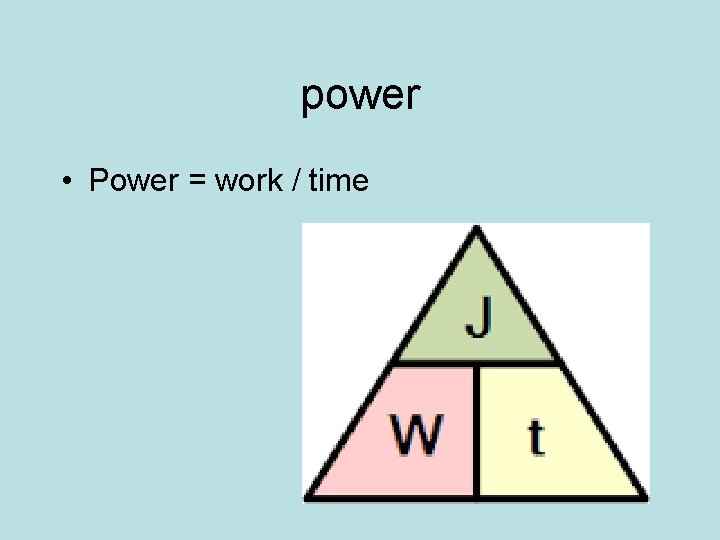
power • Power = work / time

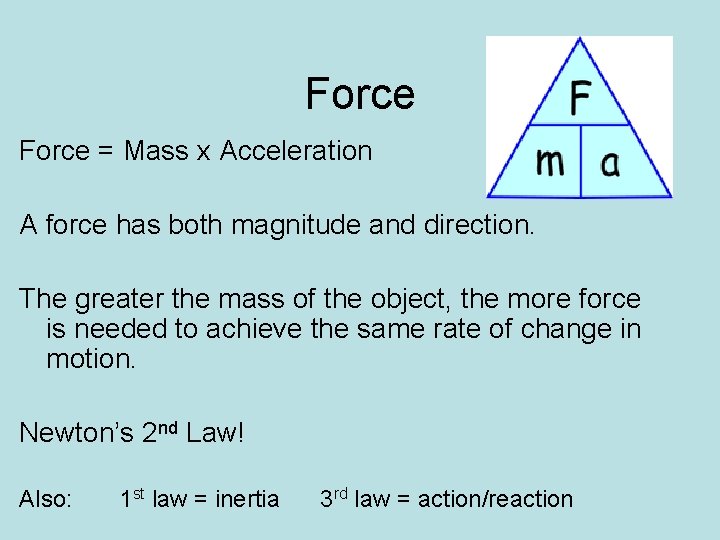
Force = Mass x Acceleration A force has both magnitude and direction. The greater the mass of the object, the more force is needed to achieve the same rate of change in motion. Newton’s 2 nd Law! Also: 1 st law = inertia 3 rd law = action/reaction

Forces There always 2 or more forces acting on static or stationary objects. Examples of these forces are: friction, gravity, elastic forces due to tension or compression of matter, normal force. Unbalanced forces create movement

Amount by which they increase force is mechanical advantage None are 100% efficient due to friction Can be combined to form compound machines