7 Aprile 2019 Viareggio LU Aspetti farmacoeconomici Claudio




- Slides: 16

7 Aprile 2019, Viareggio (LU) Aspetti farmacoeconomici Claudio Gasperini Ospedale San Camillo, Roma

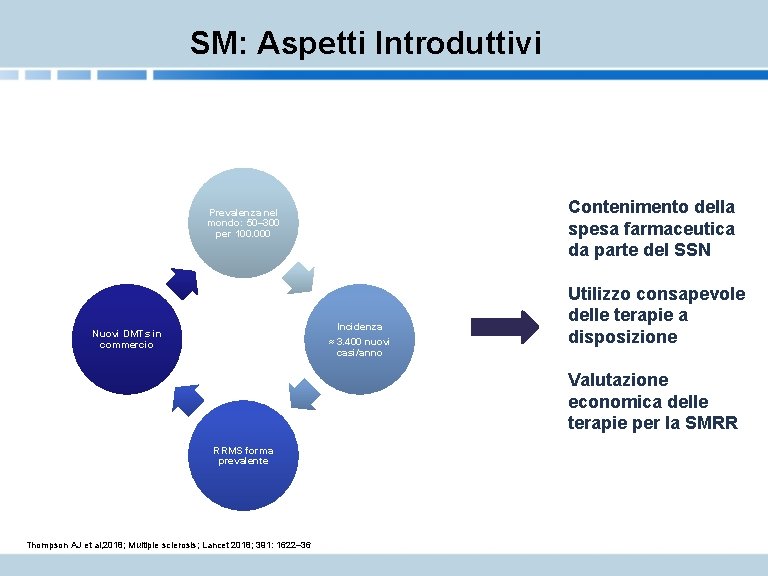
SM: Aspetti Introduttivi Contenimento della spesa farmaceutica da parte del SSN Prevalenza nel mondo: 50– 300 per 100. 000 Incidenza ≈ 3. 400 nuovi casi/anno Nuovi DMTs in commercio Utilizzo consapevole delle terapie a disposizione Valutazione economica delle terapie per la SMRR RRMS forma prevalente Thompson AJ et al, 2018; Multiple sclerosis; Lancet 2018; 391: 1622– 36

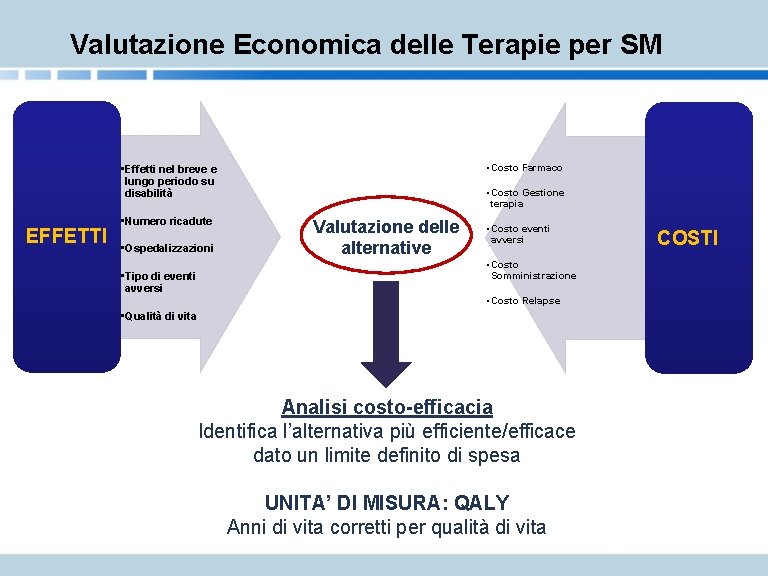
Valutazione Economica delle Terapie per SM • Costo Farmaco • Effetti nel breve e lungo periodo su disabilità EFFETTI • Numero ricadute • Ospedalizzazioni • Tipo di eventi avversi • Costo Gestione terapia Valutazione delle alternative • Costo eventi avversi • Costo Somministrazione • Costo Relapse • Qualità di vita Analisi costo-efficacia Identifica l’alternativa più efficiente/efficace dato un limite definito di spesa UNITA’ DI MISURA: QALY Anni di vita corretti per qualità di vita COSTI

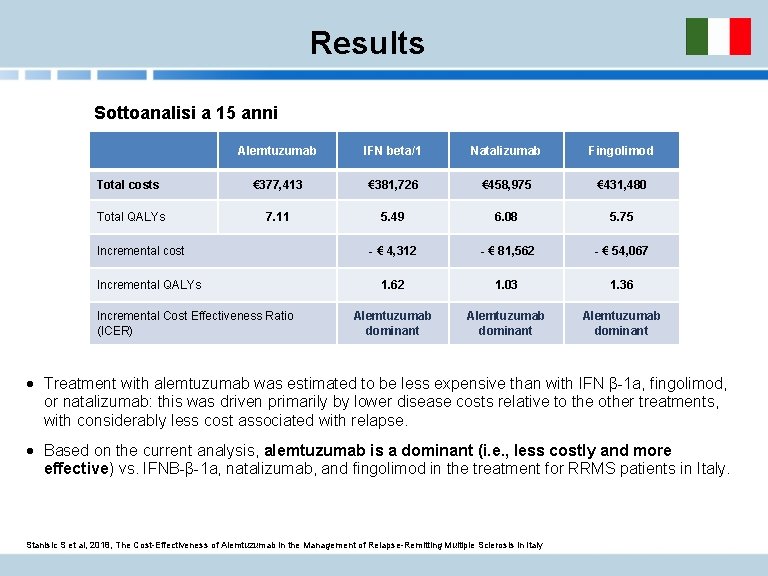
Objectives Assessing the cost-effectiveness of alemtuzumab in comparison with other disease modifying therapies (DMTs) in the management of relapsing-remitting multiple sclerosis (RRMS), from the perspective of the Italian National Health Service. Methods Markov model to assess long term clinical outcomes and costs of alemtuzumab in comparison with subcutaneous IFN β-1 a 44 µg and natalizumab and fingolimod. QALY (quality-adjusted life years ) for health effects; costs expressed in monetary units (€, 2017). All costs and health-effects were discounted at annual 3. 5% discount rate. In the absence of head-to-head data for comparators, evidence from a published network metaanalysis (NMA) was used. Data on the natural history of MS were derived from two large MS-population databases: the British Columbia MS and the London Ontario dataset. Disease outcomes were simulated using three key parameters: disability progression, incidence of relapse and mortality. Stanisic S et al, 2018, The Cost-Effectiveness of Alemtuzumab in the Management of Relapse-Remitting Multiple Sclerosis in Italy

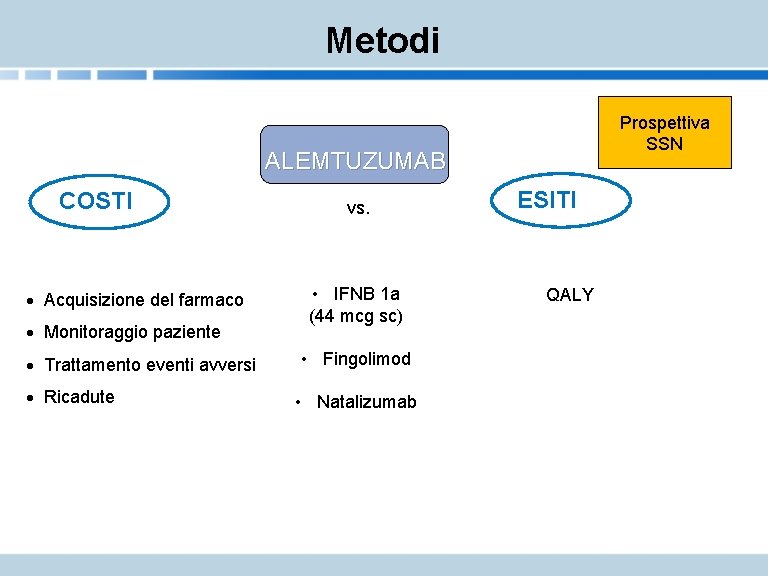
Metodi Prospettiva SSN ALEMTUZUMAB COSTI · Acquisizione del farmaco · Monitoraggio paziente · Trattamento eventi avversi · Ricadute vs. • IFNB 1 a (44 mcg sc) • Fingolimod • Natalizumab ESITI QALY

Results Sottoanalisi a 15 anni Alemtuzumab IFN beta/1 Natalizumab Fingolimod Total costs € 377, 413 € 381, 726 € 458, 975 € 431, 480 Total QALYs 7. 11 5. 49 6. 08 5. 75 - € 4, 312 - € 81, 562 - € 54, 067 1. 62 1. 03 1. 36 Alemtuzumab dominant Incremental cost Incremental QALYs Incremental Cost Effectiveness Ratio (ICER) · Treatment with alemtuzumab was estimated to be less expensive than with IFN β-1 a, fingolimod, or natalizumab: this was driven primarily by lower disease costs relative to the other treatments, with considerably less cost associated with relapse. · Based on the current analysis, alemtuzumab is a dominant (i. e. , less costly and more effective) vs. IFNB-β-1 a, natalizumab, and fingolimod in the treatment for RRMS patients in Italy. Stanisic S et al, 2018, The Cost-Effectiveness of Alemtuzumab in the Management of Relapse-Remitting Multiple Sclerosis in Italy

CONCLUSIONI · In base ai dati dello studio Italiano si stima che alemtuzumab sia meno costoso rispetto a IFNB 1 A, fingolimod e Natalizumab · Alemtuzumab risulta DOMINANTE (meno costoso e maggiormente efficace) rispetto ai farmaci studiati come competitors · Studi di farmacoeconomia condotti in Austria, Olanda e Finlandia mostrano risultati in linea con quelli dello studio Italiano

GRAZIE!

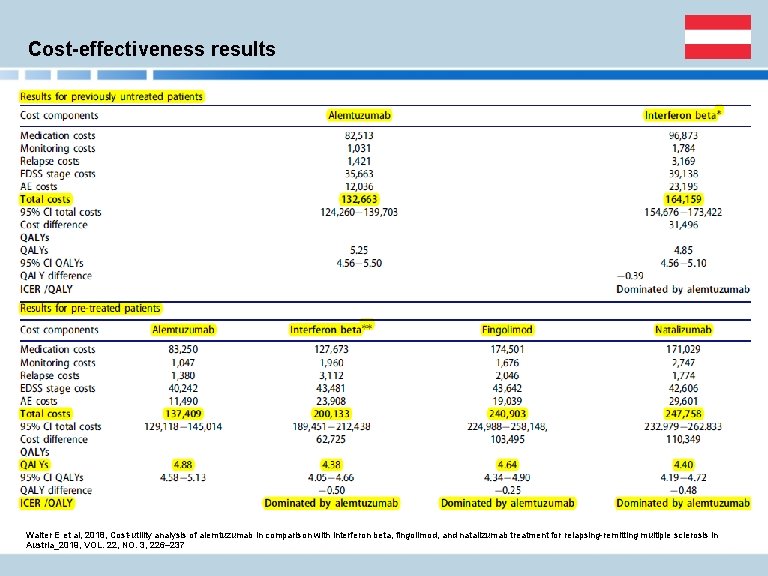
Cost-effectiveness results Walter E et al, 2018, Cost-utility analysis of alemtuzumab in comparison with interferon beta, fingolimod, and natalizumab treatment for relapsing-remitting multiple sclerosis in Austria_2019, VOL. 22, NO. 3, 226– 237

Cost-minimization analysis of alemtuzumab compared to fingolimod and natalizumab for the treatment of active relapsing-remitting multiple sclerosis in the Netherlands Objective Alemtuzumab costs analysis compared to fingolimod and natalizumab for secondline treatment of active RRMS in the Netherlands. Robustness of the base case results was verified with multiple sensitivity and scenario analyses. Methods Cost-minimization analysis performed from a Dutch healthcare perspective over a 5 -year time horizon, in order to evaluate the costs of alemtuzumab compared to fingolimod and natalizumab for second-line treatment of active RRMS. The following parameters were considered: · drug acquisition costs · administration costs · monitoring costs Assumptions regarding resource use were based on the summaries of product characteristics and hospital protocols. Unit costs were obtained from the Dutch Healthcare Authority DRG tariff databases and the Dutch costing manual. Piena MA et al, 2018, Cost-minimization analysis of alemtuzumab compared to fingolimod and natalizumab for the treatment of active relapsing-remitting multiple sclerosis in the Netherlands, Journal of Medical Economics, 21: 10, 968 -976

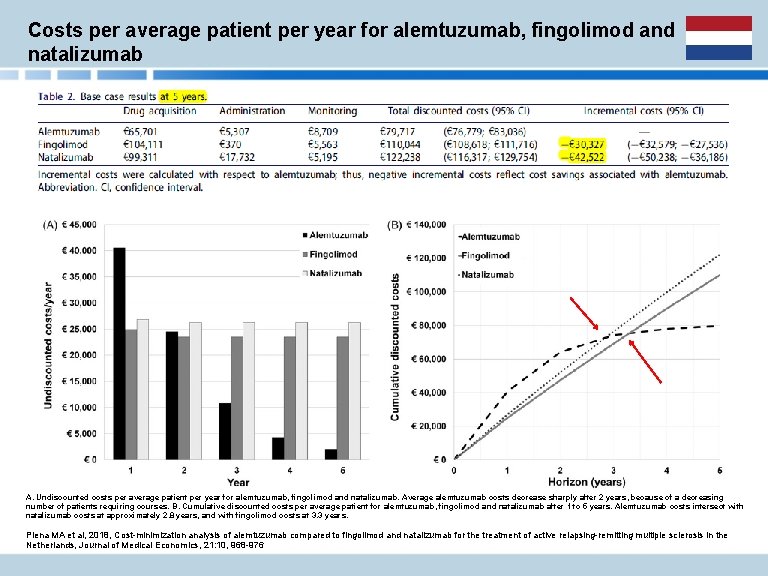
Costs per average patient per year for alemtuzumab, fingolimod and natalizumab A. Undiscounted costs per average patient per year for alemtuzumab, fingolimod and natalizumab. Average alemtuzumab costs decrease sharply after 2 years, because of a decreasing number of patients requiring courses. B. Cumulative discounted costs per average patient for alemtuzumab, fingolimod and natalizumab after 1 to 5 years. Alemtuzumab costs intersect with natalizumab costs at approximately 2. 8 years, and with fingolimod costs at 3. 3 years. Piena MA et al, 2018, Cost-minimization analysis of alemtuzumab compared to fingolimod and natalizumab for the treatment of active relapsing-remitting multiple sclerosis in the Netherlands, Journal of Medical Economics, 21: 10, 968 -976

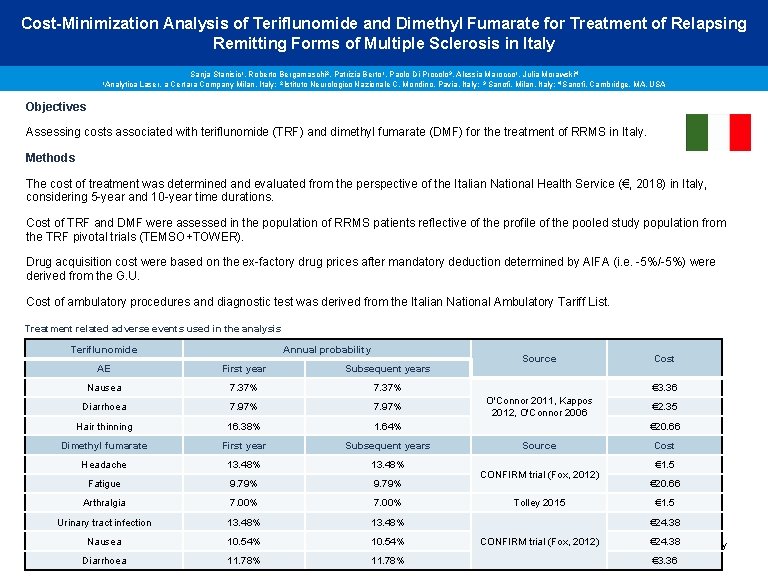
Cost-Minimization Analysis of Teriflunomide and Dimethyl Fumarate for Treatment of Relapsing Remitting Forms of Multiple Sclerosis in Italy 1 Analytica Sanja Stanisic 1, Roberto Bergamaschi 2, Patrizia Berto 1, Paolo Di Procolo 3, Alessia Marocco 1, Julia Morawski 4 Laser, a Certara Company Milan, Italy; 2 Istituto Neurologico Nazionale C. Mondino, Pavia, Italy; 3 Sanofi, Milan, Italy; 4 Sanofi, Cambridge, MA, USA Objectives Assessing costs associated with teriflunomide (TRF) and dimethyl fumarate (DMF) for the treatment of RRMS in Italy. Methods The cost of treatment was determined and evaluated from the perspective of the Italian National Health Service (€, 2018) in Italy, considering 5 -year and 10 -year time durations. Cost of TRF and DMF were assessed in the population of RRMS patients reflective of the profile of the pooled study population from the TRF pivotal trials (TEMSO+TOWER). Drug acquisition cost were based on the ex-factory drug prices after mandatory deduction determined by AIFA (i. e. -5%/-5%) were derived from the G. U. Cost of ambulatory procedures and diagnostic test was derived from the Italian National Ambulatory Tariff List. Treatment related adverse events used in the analysis Teriflunomide Annual probability AE First year Subsequent years Nausea 7. 37% Diarrhoea 7. 97% Hair thinning 16. 38% 1. 64% Dimethyl fumarate First year Subsequent years Headache 13. 48% Fatigue 9. 79% Arthralgia 7. 00% Urinary tract infection 13. 48% Source Cost € 3. 36 O'Connor 2011, Kappos 2012, O'Connor 2006 € 2. 35 € 20. 66 Source CONFIRM trial (Fox, 2012) Tolley 2015 Cost € 1. 5 € 20. 66 € 1. 5 € 24. 38 CONFIRM trial (Fox, 2012) 10. 54% € 24. 38 Stanisic S et al, Nausea 2018. Hamidi et al, Cost-Minimization Analysis of Teriflunomide and Dimethyl Fumarate for Treatment of Relapsing Remitting Forms of Multiple Sclerosis in Italy Diarrhoea 11. 78% € 3. 36

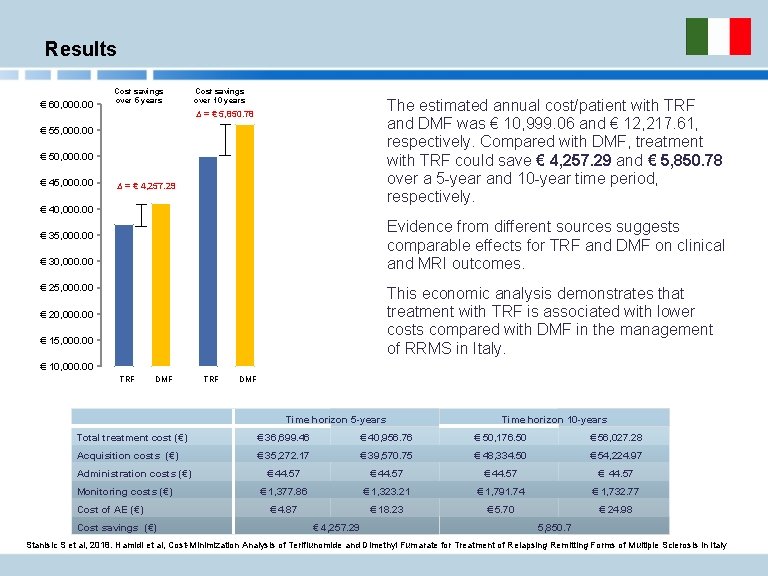
Results € 60, 000. 00 Cost savings over 5 years Cost savings over 10 years The estimated annual cost/patient with TRF and DMF was € 10, 999. 06 and € 12, 217. 61, respectively. Compared with DMF, treatment with TRF could save € 4, 257. 29 and € 5, 850. 78 over a 5 -year and 10 -year time period, respectively. ∆ = € 5, 850. 78 € 55, 000. 00 € 50, 000. 00 € 45, 000. 00 ∆ = € 4, 257. 29 € 40, 000. 00 Evidence from different sources suggests comparable effects for TRF and DMF on clinical and MRI outcomes. € 35, 000. 00 € 30, 000. 00 € 25, 000. 00 This economic analysis demonstrates that treatment with TRF is associated with lower costs compared with DMF in the management of RRMS in Italy. € 20, 000. 00 € 15, 000. 00 € 10, 000. 00 TRF DMF Time horizon 5 -years Time horizon 10 -years Total treatment cost (€) € 36, 699. 46 € 40, 956. 76 € 50, 176. 50 € 56, 027. 28 Acquisition costs (€) € 35, 272. 17 € 39, 570. 75 € 48, 334. 50 € 54, 224. 97 € 44. 57 € 1, 377. 86 € 1, 323. 21 € 1, 791. 74 € 1, 732. 77 € 4. 87 € 18. 23 € 5. 70 € 24. 98 Administration costs (€) Monitoring costs (€) Cost of AE (€) Cost savings (€) € 4, 257. 29 5, 850. 7 Stanisic S et al, 2018. Hamidi et al, Cost-Minimization Analysis of Teriflunomide and Dimethyl Fumarate for Treatment of Relapsing Remitting Forms of Multiple Sclerosis in Italy

Cost-utility of First-line Disease-modifying Treatments for Relapsing– Remitting Multiple Sclerosis Objective Evaluation of the cost-effectiveness of first-line treatments of RRMS: TRF, DMF, glatiramer acetate IFN]-β 1 a 44 µg TIW, IFN-β 1 b 250 µg and IFN-β 1 a 30 µg and best supportive care (BSC) in the health care payer setting in Finland Methods The primary outcome was the modeled incremental cost-effectiveness ratio (ICER; €/quality adjusted life-year [QALY] gained, 3%/y discounting). Markov cohort modeling with a 15 -year time horizon was employed. Patients‘ characteristics, RRMS progression probabilities, and standardized mortality ratios were derived from a registry of patients with MS in Finland. Four approaches were used to assess the outcomes: · cost-effectiveness plane and efficiency frontiers; · cost-effectiveness acceptability frontier, which demonstrated optimal treatment to maximize net benefit; · Bayesian treatment ranking (BTR); · an impact investment Assessment (IIA; a cost-benefit assessment), which increased the clinical interpretation and appeal of modeled outcomes in terms of absolute benefit gained with fixed drug-related budget; Robustness of results was tested extensively with sensitivity analyses. Soini, S et al, Cost-utility of. First-line. Disease-modifying Treatments for. Relapsing–Remitting Multiple Sclerosis; Clinical Therapeutics/Volume 39, Number 3, 2017

Costs per average patient per year for alemtuzumab, fingolimod and natalizumab The findings from this modeling study of firstline DMTs for RRMS over 15 years suggest that teriflunomide 14 mg saves costs in comparison with all other reimbursed first-line DMTs in Finland; Is cost-effective in comparison with DMF 240 mg at the cited threshold values; Dominates injectable first-line DMTs; and, as a complementary result, is associated with the most value gained versus BSC as per the limited DMT-related budget(WTI). BSC = best supportive care Soini, S et al, Cost-utility of. First-line. Disease-modifying Treatments for. Relapsing–Remitting Multiple Sclerosis; Clinical Therapeutics/Volume 39, Number 3, 2017

CONCLUSIONI · Da compilare
 7 aprile 2019
7 aprile 2019 Istituto comprensivo motto viareggio
Istituto comprensivo motto viareggio Centro pma versilia
Centro pma versilia Rossi aprile
Rossi aprile 20 4 1889
20 4 1889 Contesto extralinguistico
Contesto extralinguistico Prova scritta tfa sostegno primaria
Prova scritta tfa sostegno primaria Avanzo fusione
Avanzo fusione Aspetti verbali
Aspetti verbali Organizzazione di significato personale
Organizzazione di significato personale Internet aspetti positivi e negativi
Internet aspetti positivi e negativi Spadon claudio
Spadon claudio Luci sapienza
Luci sapienza Claudio storelli
Claudio storelli Claudio marchesano
Claudio marchesano Colegio claudio matte temuco
Colegio claudio matte temuco Claudio cumani
Claudio cumani