5 3 Organic Compounds organic compounds always contain

- Slides: 11

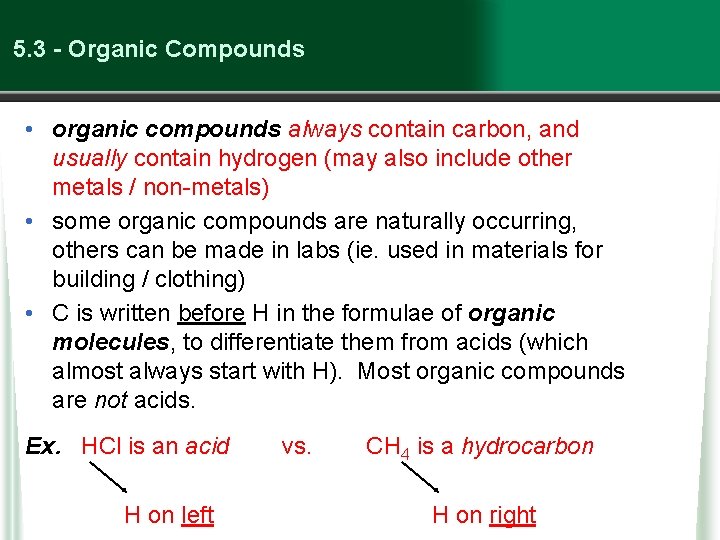
5. 3 - Organic Compounds • organic compounds always contain carbon, and usually contain hydrogen (may also include other metals / non-metals) • some organic compounds are naturally occurring, others can be made in labs (ie. used in materials for building / clothing) • C is written before H in the formulae of organic molecules, to differentiate them from acids (which almost always start with H). Most organic compounds are not acids. Ex. HCl is an acid H on left vs. CH 4 is a hydrocarbon H on right

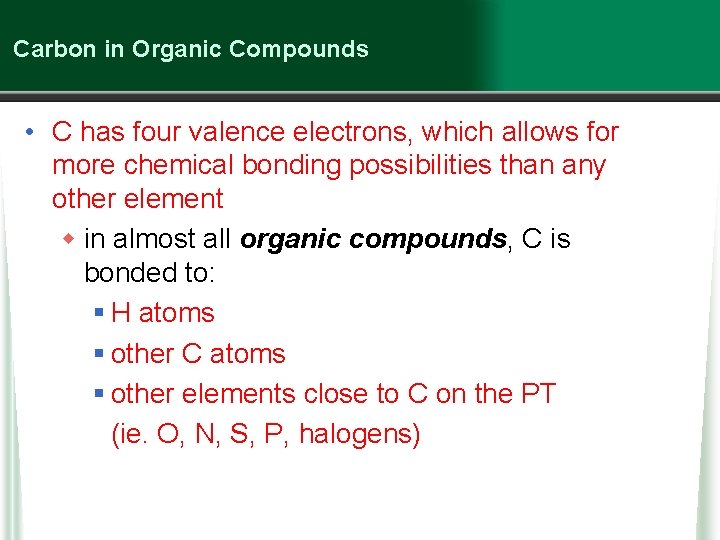
Carbon in Organic Compounds • C has four valence electrons, which allows for more chemical bonding possibilities than any other element w in almost all organic compounds, C is bonded to: § H atoms § other C atoms § other elements close to C on the PT (ie. O, N, S, P, halogens)

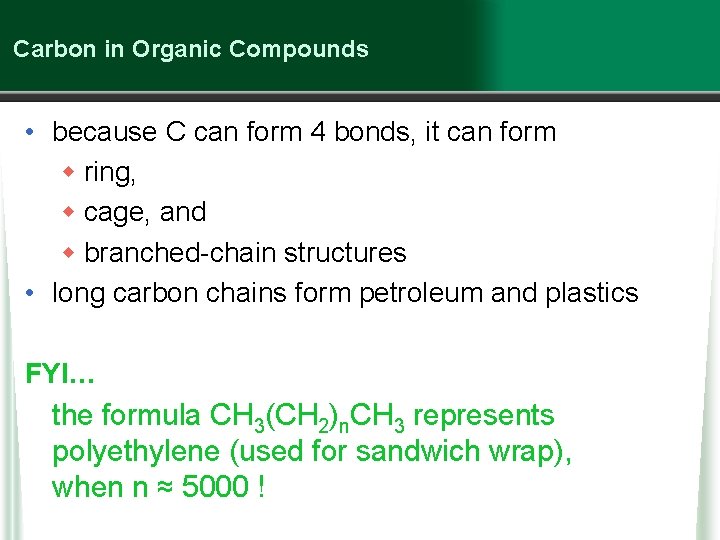
Carbon in Organic Compounds • because C can form 4 bonds, it can form w ring, w cage, and w branched-chain structures • long carbon chains form petroleum and plastics FYI… the formula CH 3(CH 2)n. CH 3 represents polyethylene (used for sandwich wrap), when n ≈ 5000 !

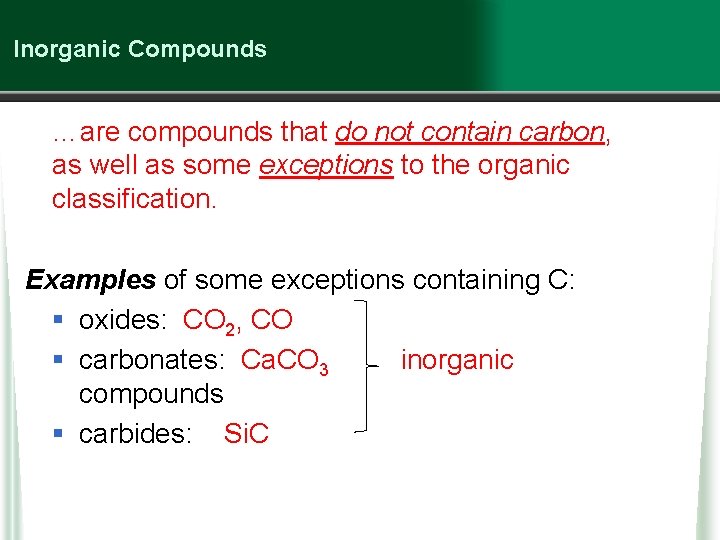
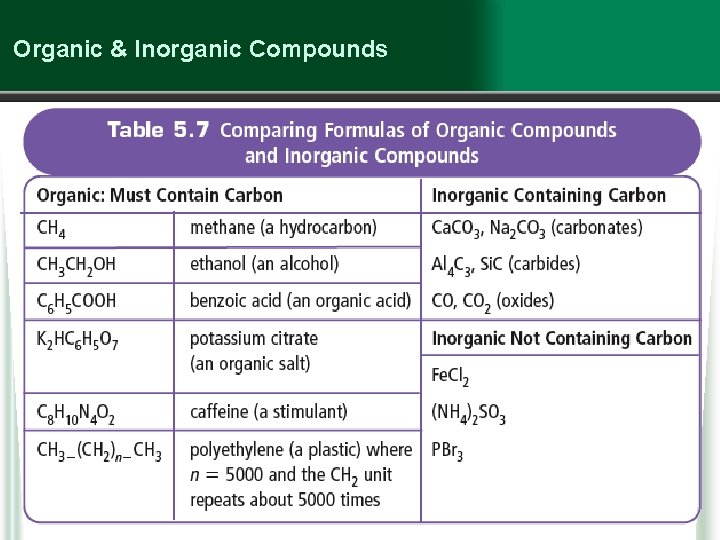
Inorganic Compounds …are compounds that do not contain carbon, as well as some exceptions to the organic classification. Examples of some exceptions containing C: § oxides: CO 2, CO § carbonates: Ca. CO 3 inorganic compounds § carbides: Si. C

Organic & Inorganic Compounds

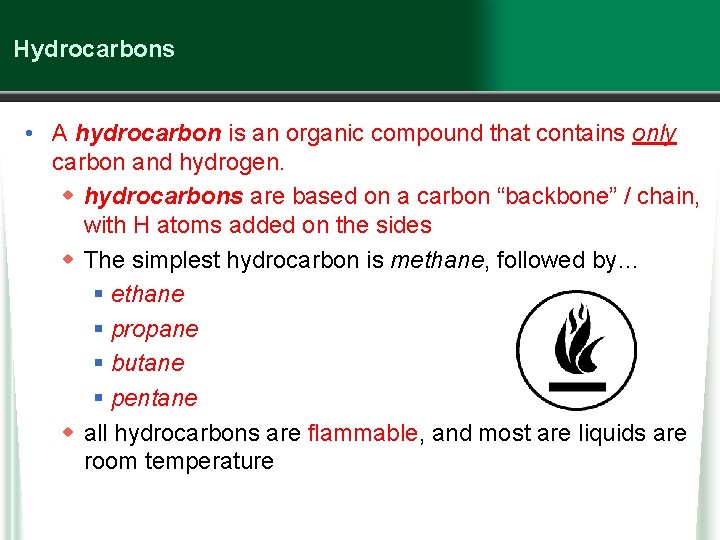
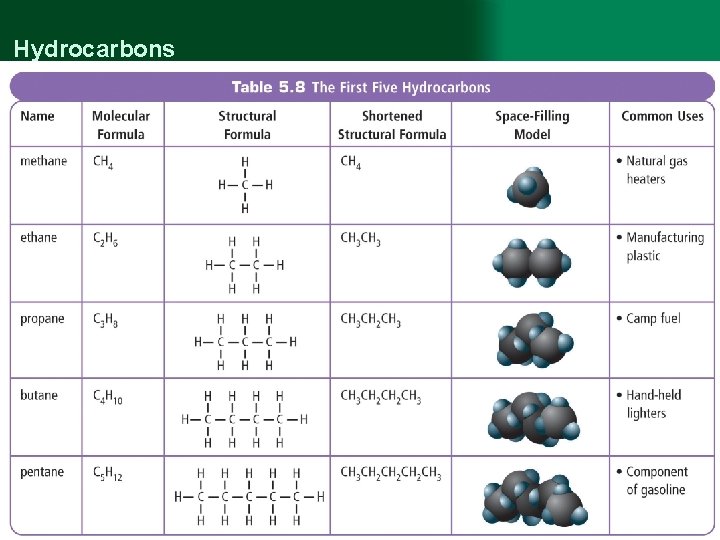
Hydrocarbons • A hydrocarbon is an organic compound that contains only carbon and hydrogen. w hydrocarbons are based on a carbon “backbone” / chain, with H atoms added on the sides w The simplest hydrocarbon is methane, followed by… § ethane § propane § butane § pentane w all hydrocarbons are flammable, and most are liquids are room temperature

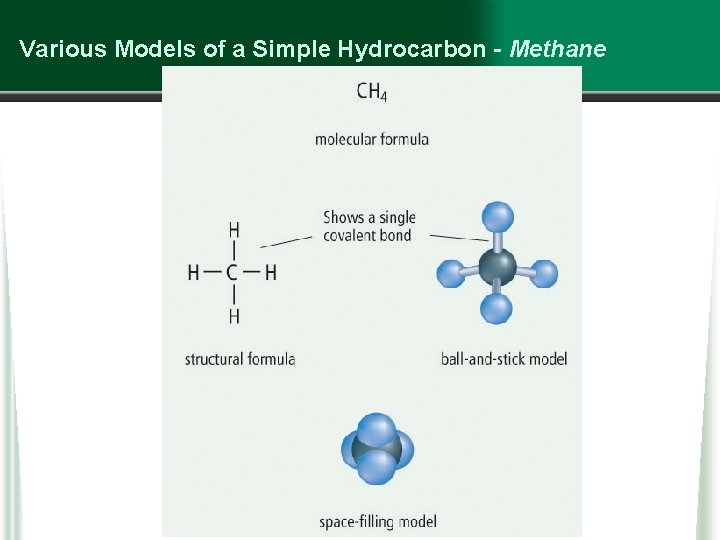
Various Models of a Simple Hydrocarbon - Methane

Hydrocarbons

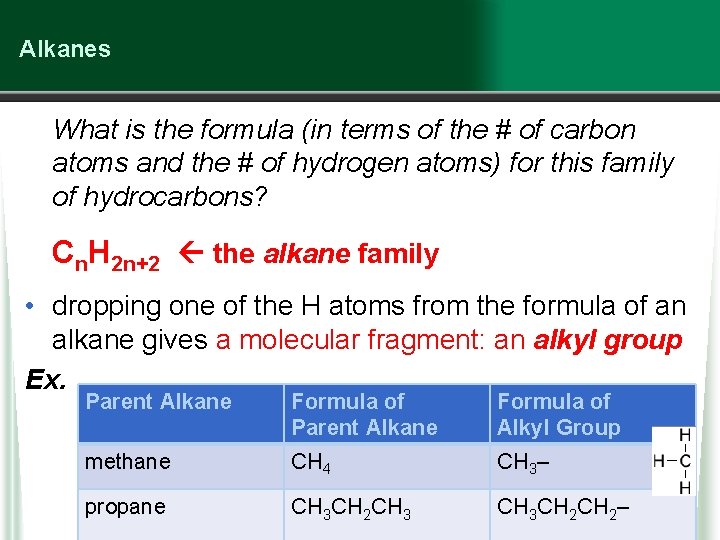
Alkanes What is the formula (in terms of the # of carbon atoms and the # of hydrogen atoms) for this family of hydrocarbons? Cn. H 2 n+2 the alkane family • dropping one of the H atoms from the formula of an alkane gives a molecular fragment: an alkyl group Ex. Parent Alkane Formula of Alkyl Group methane CH 4 CH 3– propane CH 3 CH 2 CH 3 CH 2–

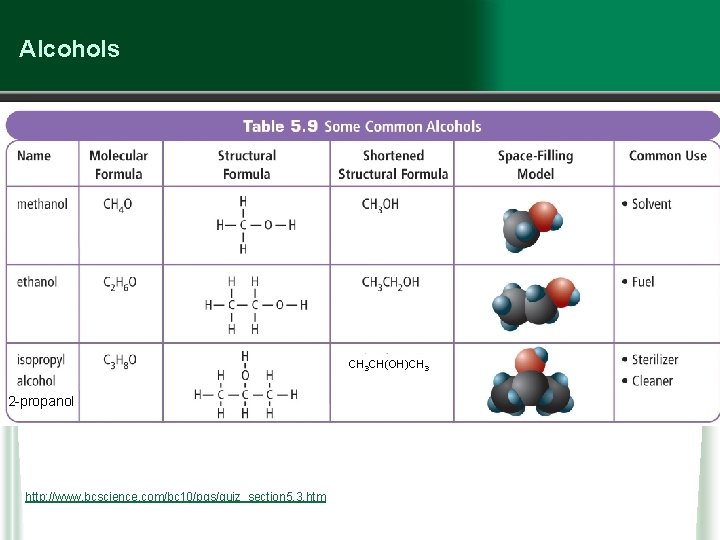
Alcohols • alcohols are organic compounds containing C, H and O • H and O are “packaged” as a hydroxyl (–OH) group (Note: this is not the same as a hydroxide ion) • alcohols are organic compounds with at least one –OH group attached to an alkyl group w the simplest alcohols are methanol (CH 4 O), ethanol (C 2 H 6 O) and isopropyl alcohol (C 3 H 8 O) w alcohols are very good solvents, and are generally very flammable

Alcohols CH 3 CH(OH)CH 3 2 -propanol http: //www. bcscience. com/bc 10/pgs/quiz_section 5. 3. htm