3633 Central Avenue Ste I Hot Springs AR
























- Slides: 32


3633 Central Avenue, Ste. I • Hot Springs, AR 71913 Phone: (501) 542 -4052 • Fax: (501) 321 -2886 E-mail: info@burkepharmaceutical. com • Website: www. burkepharmaceutical. com

Statements under the Private Securities Litigation Reform Act: With the exception of the historical information contained in this release, the matters described herein contain forwardlooking statements that involve risk and uncertainties that may individually or mutually impact the matters herein described, including but not limited to its ability to enter into partnering agreements or raise financing on acceptable terms, successful completion of clinical development programs, regulatory review and approval, product development and acceptance, manufacturing, competition, and/or other factors, some of which are outside the control of the Company.

o To develop innovative dermatology products for unmet medical needs: • • • Develop new indications for approved drugs Develop New Delivery Systems Develop improved formulations Rx or OTC (NDA, ANDA, monograph products) Employ 505(b)(2) NDA regulatory strategy

Burke Pharmaceuticals, Inc. is dedicated to delivering innovative dermatology products. Novel delivery systems, superior formulations and strategic planning by an experienced team are priority. Convenient products will address unmet medical needs for both patients and physicians.

Privately Held Incorporated Shares Outstanding Capital Infusion Founded Number of Employees Sister Companies • Arkansas • N/A • $3 Million • 2008 • 6 • Burke Pharmaceutical Research, Inc. • The Dermatology Clinic

Management Team Dr. Dow Stough, MD Tim Dugan William Pfister, Ph. D. • 20+ years practicing dermatologist • Founder of three companies • Expertise in clinical trials, product development, and treatment of skin diseases • Managing Director • Responsible for operations, financing, administration • Early Drug Development • Scientific Advisor

Dowling B. Stough M. D. , F. A. A. D. , C. C. T. I. • Dr. Stough is the founder and practicing dermatologist of The Dermatology Clinic, P. A. and medical director of Burke Pharmaceutical Research. Founded in 2000, Burke Pharmaceutical has grown from a small clinical trials unit within a dermatology practice to a national leader in clinical trials of skin disease. Currently, it employs an experienced site manager, three clinical coordinators, a full time advanced nurse practitioner and three physician investigators. Over the last 10 years, Burke Pharmaceutical Research has been involved in over ninety clinical studies. Dr. Stough has served as the medical director for various pharmaceutical companies conducting clinical trials, and has conducted multiple investigator-initiated studies. • Dr. Stough holds patents on a medical device known as the “Wart Eraser. ” He has also been instrumental in developing other medical devices. • Dr. Stough is the founder and past president of the International Society of Hair Restoration Surgery. He was the author of two leading textbooks dedicated to hair restoration surgery. He has written over 50 scientific publications and has contributed numerous textbook chapters. He has presented over 180 medical lectures at scientific meetings and was the recipient of the “Golden Follicle Award” by the International Society of Hair Restoration Surgery. He is a fellow of the American Academy of Dermatology and holds a certified physician investigator designation from the Association of Clinical Research Professionals. His interest in dermatologic drug development is preceded by over 15 years of hands on exposure to clinical trials and protocol development.

Dowling B. Stough M. D. , F. A. A. D. , C. C. T. I. � Dr. Stough has always had an interest in research, after his medicine internship at Baptist Hospital in Memphis, Tennessee; he was involved in research during his dermatology residency at the University of Arkansas Medical School. Following this, Dr. Stough was accepted into a one year fellowship sponsored by the American Academy of Cosmetic Surgery. After maintaining an active dermatology practice for 20 years, he recently divested his practice to devote full time attention to clinical research and drug development. He continues as Clinical Assistant Professor of Dermatology at the University of Arkansas for Medical Sciences. He maintains active licenses in Arkansas, Florida, Texas, Tennessee, and New York and continues to see research related patients. � BURKE Pharmaceutical is dedicated to the commercial development of pharmaceutical products and will draw upon the experience and knowledge gained thru years of research and medical practice. The targeted products will be physician driven and will fill voids that have not been currently addressed by other pharmaceutical companies.

Tim Dugan, BS, CCRC � Mr. Dugan has over 11 years of experience in clinical research. He has developed a large database of contacts with key personnel in the industry. Seven years ago, he became the director of clinical operations for Burke Pharmaceutical Research. As director, Mr. Dugan handles all the clinical and regulatory aspects involved in the trial studies; oversees all assisting personnel, negotiates contracts and budgets, and supervises subject conduct during the trial. He is also instrumental in feasibility studies in the early development of proprietary drugs initiated by BLU Pharmaceutical. � After graduation from the University of Arkansas, he began his career in clinical trial research at Physicians Group Research Clinic. While employed, he successfully attained his certificate as a Certified Clinical Research Coordinator. He then became the regional director of Clinical Operations for Research Solutions, a national site management organization based in Little Rock, Arkansas. He served as the director overseeing 10 clinical research sites and managed a dozen research coordinators. � Mr. Dugan received a B. S. in Biology from the University of Arkansas. He also holds certifications from the Association of Clinical Research Professionals, Certified Clinical Research Coordinator (CCRC), Model Agreement Group Initiative, and Clinical Research Contract Professional (CRCP).

William R. Pfister, Ph. D. � Ph. D. in Pharmacology and Toxicology. � Senior level executive with 30 years of pharmaceutical company experience in: quality control, technical service, product development, preclinical and clinical affairs, regulatory affairs & quality assurance, project management, scientific affairs and business development. � Held senior management positions and supported pharmaceutical product research and development at Hoffmann La-Roche, American Critical Care, Dow Corning Corporation, Phar. Metrix Corporation, Lavipharm Laboratories, Nex. Med USA, NAL Pharmaceuticals and BLU Pharmaceuticals, Inc. � Expertise in topical, transdermal, parenteral products and controlled release systems. Developed regulatory strategy and authored and filed numerous INDs and amendments (CMC, preclinical and clinical), NDAs and e. CTDs. � Certified Regulatory Affairs Professional (RAC), Diplomate of the American Board of Toxicology (DABT), and is author of several patents, publications and books including: “Transdermal and Topical Drug Delivery Systems, (1997) and “Intraoral Drug Delivery: Molecules to Market” (2005).

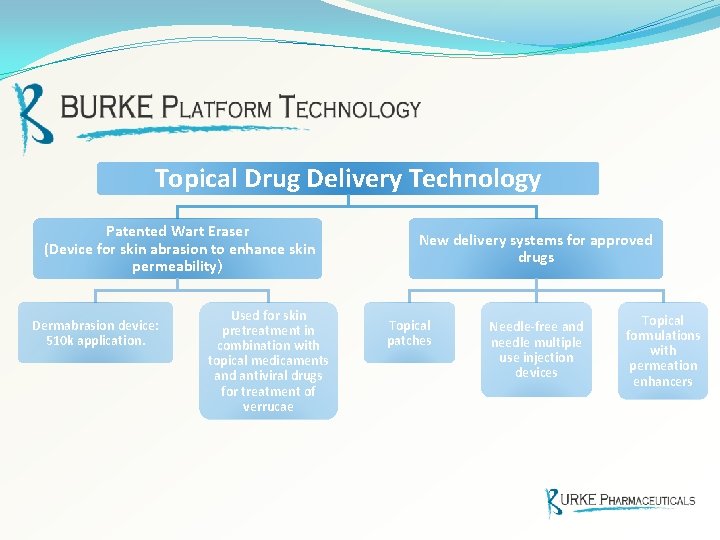
Topical Drug Delivery Technology Patented Wart Eraser (Device for skin abrasion to enhance skin permeability) Dermabrasion device: 510 k application. Used for skin pretreatment in combination with topical medicaments and antiviral drugs for treatment of verrucae New delivery systems for approved drugs Topical patches Needle-free and needle multiple use injection devices Topical formulations with permeation enhancers

1. Novel Rx Dermatology Products: Off-Patent Drugs: • Known Efficacy & Safety • No Topical Dosage Form 505 (b)(2) New Drug Products: • New Route/Indication • New Dosage Form 2. Improved OTC Dermatology Products: Off-Patent Drugs: • Monograph Drugs • Rx to OTC Switch Drugs OTC 505 (b)(2) Monograph New Drug Products: • Extended Brand Life • Extend Patent Life 505 (b) (2) strategy - Between 2005 and 2015 patents expire on drugs having more than $150 billion in sales!

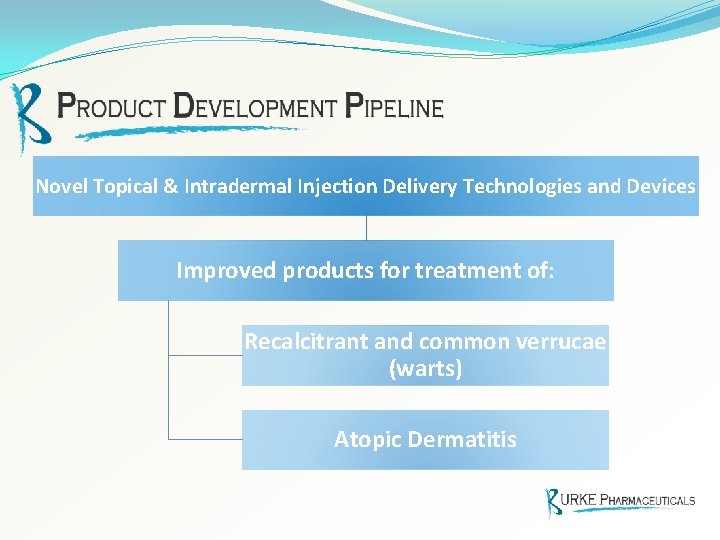
Novel Topical & Intradermal Injection Delivery Technologies and Devices Improved products for treatment of: Recalcitrant and common verrucae (warts) Atopic Dermatitis

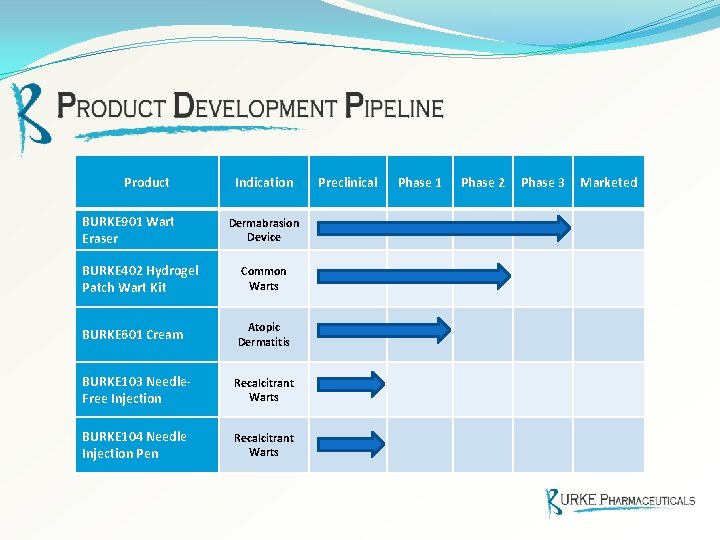
Product BURKE 901 Wart Eraser Indication Dermabrasion Device BURKE 402 Hydrogel Patch Wart Kit Common Warts BURKE 601 Cream Atopic Dermatitis BURKE 103 Needle. Free Injection Recalcitrant Warts BURKE 104 Needle Injection Pen Recalcitrant Warts Preclinical Phase 1 Phase 2 Phase 3 Marketed

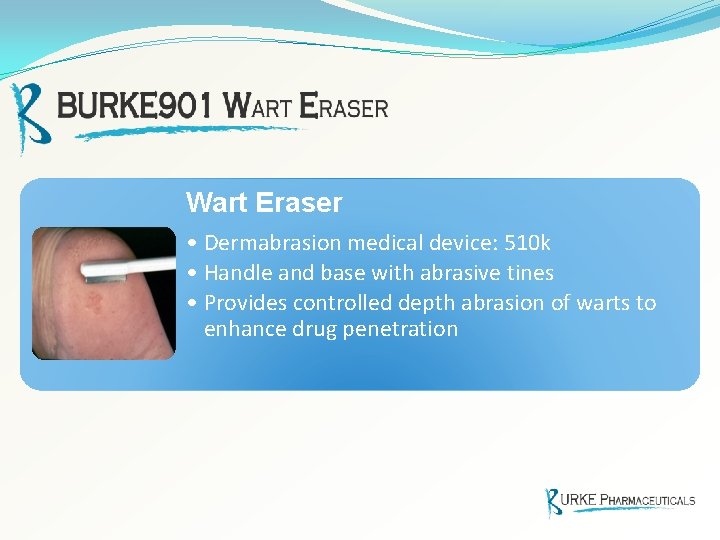
Wart Eraser • Dermabrasion medical device: 510 k • Handle and base with abrasive tines • Provides controlled depth abrasion of warts to enhance drug penetration

Patent Portfolio: 2 patents & 3 Applications 1. Stough, DB, Wart Removal Method and Device, US Patent Application No. US 002/0169462 A 1, published November 14, 2004. 2. Stough, DB, Wart Removal Method and Device, US Patent 6, 585, 742, B 2, issued July 1, 2003. 3. Stough, DB, Wart Removal Method and Device, US Patent Application No. US 004/0138679 A 1, published July 15, 2004. 4. Stough, DB, Wart Removal Method and Device, US Patent 7, 056, 324, B 2, issued June 6, 2006. 5. Stough, DB, Wart Removal method and Device, US Patent Application No. US 2006/0200174 A 1, published September 7, 2006. Wart Eraser

BURKE 402 Hydrogel Patch Wart Kit • Abrade Skin with Wart Eraser • Apply patch to wart • Daily application for up to 6 weeks BURKE 601 Cream • Co-license agreement signed 1 Q 2010 between Cypress Pharmaceutical, Inc. and BLU Pharmaceuticals, Inc. to develop prescription product for atopic dermatitis • Pre-IND meeting March 2010

BURKE 103 Needle-free Injection • Reconstitute lyophilized drug with sterile saline • Needleless intradermal injection BURKE 104 Needle Injection Pen • Reconstitute lyophilized drug with sterile saline • Needle intradermal injection

Wart Prevalence • Cutaneous warts are extremely prevalent with a frequency of 20% in school age children. • After childhood the prevalence decreases with increasing age but still maintains over 5% of the population. • Ranks among the 3 most common dermatosis. Source: Jean Bologania 2 nd edition Dermatology pg. 1183.

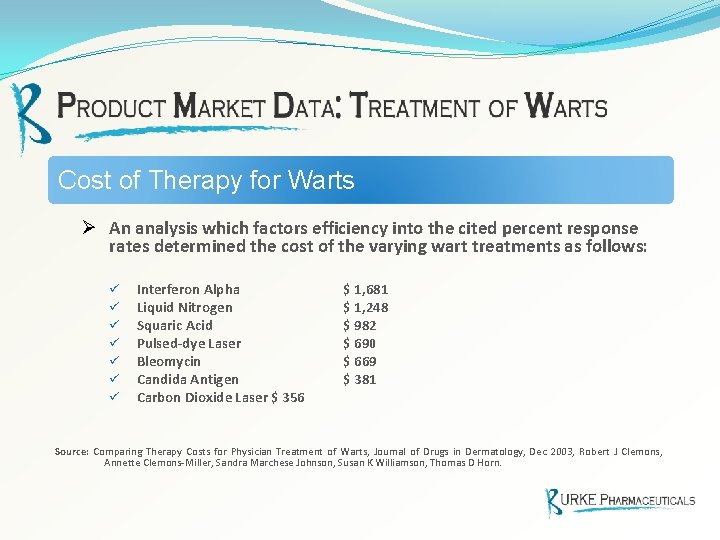
Cost of Therapy for Warts Ø An analysis which factors efficiency into the cited percent response rates determined the cost of the varying wart treatments as follows: ü ü ü ü Interferon Alpha Liquid Nitrogen Squaric Acid Pulsed-dye Laser Bleomycin Candida Antigen Carbon Dioxide Laser $ 356 $ 1, 681 $ 1, 248 $ 982 $ 690 $ 669 $ 381 Source: Comparing Therapy Costs for Physician Treatment of Warts, Journal of Drugs in Dermatology, Dec 2003, Robert J Clemons, Annette Clemons-Miller, Sandra Marchese Johnson, Susan K Williamson, Thomas D Horn.

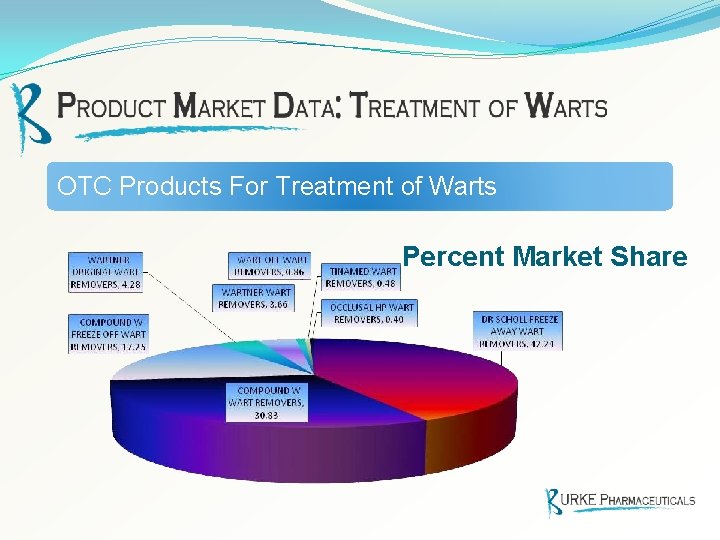
OTC Products For Treatment of Warts Percent Market Share

There are no FDA approved Rx products for treatment of warts (except anogenital warts) and OTC products are generally ineffective. There remains an unmet medical need for products that are consumer friendly, easy to use, painfree and effective for treatment of: • Common solitary warts on the trunk and extremities (excluding mucous membranes and genital warts). • Solitary lesions on the foot or hand. • Mosaic pattern warts (> 3 lesions grouped closely together). • Periungual warts (adjacent to the nail fold or distal nail plate). • Resistant or Recalcitrant warts (warts which have failed to respond to previous treatments).

Treatment of Warts Competition Marketed Products Brand (Active) OTC Salicylic Acid Preparations OTC Non. RX Freezing Duct Tape Rx Freezing Aldara (Imiquimod 5% Cream) Condolyx (Podofilox 0. 5% Gel) Minor Surgery Blenoxane (Bleomycin) Cantharidin Efficacy Minimal Somewhat Minimal Good Moderate (anogenital warts only) Good Injection under wart Topical Under Occlusion NO NO Dosage Forms Drops, Gels, Pads, and Plasters Temp. of 70 F or - 57 C Occlusion “suffocation ” Temp. of - 320 F or 196 C Cream Gel Cutting, Pairing, Electrodessication and Curettage, Laser FDA Approved YES NO YES YES N/A

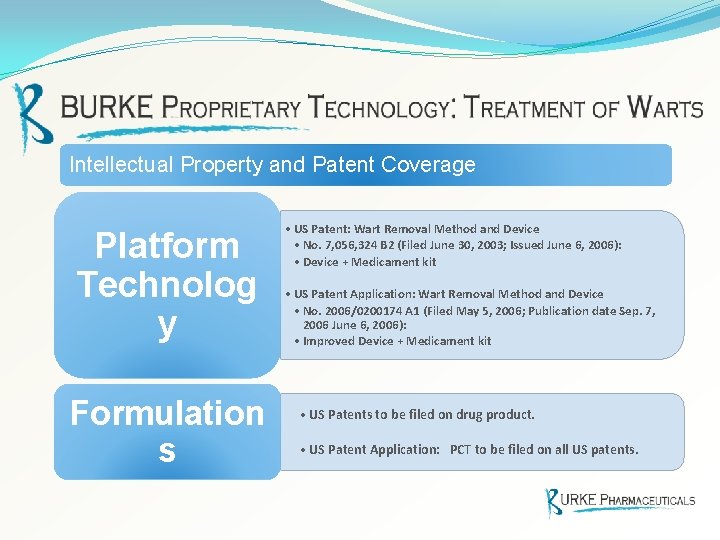
Intellectual Property and Patent Coverage Platform Technolog y Formulation s • US Patent: Wart Removal Method and Device • No. 7, 056, 324 B 2 (Filed June 30, 2003; Issued June 6, 2006): • Device + Medicament kit • US Patent Application: Wart Removal Method and Device • No. 2006/0200174 A 1 (Filed May 5, 2006; Publication date Sep. 7, 2006 June 6, 2006): • Improved Device + Medicament kit • US Patents to be filed on drug product. • US Patent Application: PCT to be filed on all US patents.

Advantages Over Marketed Products • Low Target intradermal dose per wart = 1 unit (1 mg). • Effective for treatment of recalcitrant warts. • No approved Rx products for common warts: first line therapy and superior to OTC products (salicylic acid). • Current standard of care: surgery, cryoablation, etc. • “Stone age” treatment, painful, costly, poor patient acceptance.

BLU 103/104 Development Studies • Preclinical Formulation Development & Stability (Cirrus Pharmaceuticals) • Human Skin In-Vitro Permeation and Absorption Studies (Cirrus Pharmaceuticals) • Rabbit Skin Irritation (Eurofins – Product Safety Laboratory) • Rabbit Papilloma Model (Neil Christensen, Ph. D – Penn State University) • Clinical Pilot Study (Dow Stough, MD – Burke Pharmaceutical Research)

BLU 103/104 Development Plan • Pre-IND meeting in 2 Q 2010 • File IND in 2 Q 2010 • Initiate Pilot Phase 2 a safety and Efficacy Study 2 Q 2010 • Initiate Phase 2 b safety and Efficacy Study: 3 Q 2010 • Hold EOP 2 meeting with the FDA, 3 Q 2010 • Initiate one well controlled Phase 3 studies, 4 Q 2010 • Complete Phase 3, 1 Q 2011 • File 505(b)(2) NDA, 2 Q 2011

Conclusions • In-vitro drug permeation studies on human abraded skin demonstrate target dermal drug concentrations can be achieved. • The dose of drug per wart is approximately 1 mg per application. • BURKE 101 Patch Kit or injection is expected to have a superior safety profile. • BURKE 103/104 injection (needle-free or needle) has compelling clinical data to support efficacy and are on a parallel development program.

BURKE is Seeking • Funding for pipeline products • Co-development partnerships • Venture capital investment • Licensing and marketing partners

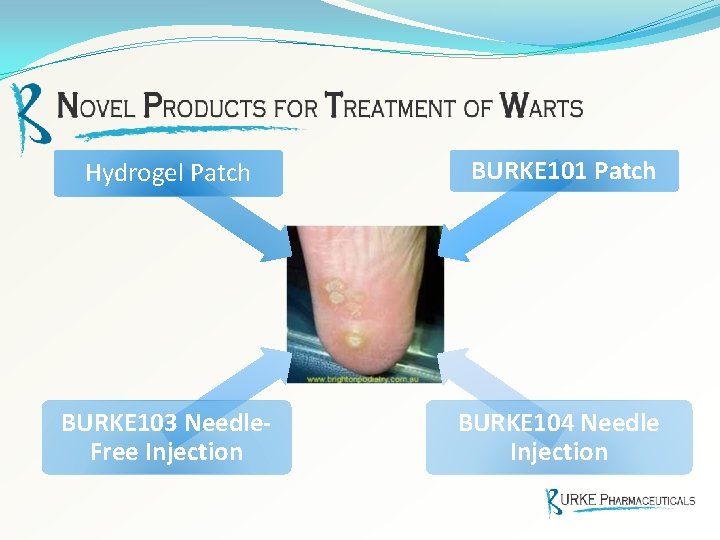
Hydrogel Patch BURKE 101 Patch BURKE 103 Needle. Free Injection BURKE 104 Needle Injection

Thank You for Your Attention!
 3633 central avenue
3633 central avenue Hot nor
Hot nor White hot vs red hot temperature
White hot vs red hot temperature Advantage of hot working process
Advantage of hot working process Perbedaan hot lava dan hot lava volcano
Perbedaan hot lava dan hot lava volcano Ifsi sainte marguerite
Ifsi sainte marguerite All languages are equally complex
All languages are equally complex 777 indiana avenue
777 indiana avenue 2829 elysian fields avenue
2829 elysian fields avenue Sten pisang
Sten pisang Nogdawindamin
Nogdawindamin Megavideo x company
Megavideo x company Paint nite sault ste marie
Paint nite sault ste marie Moonrunners the warriors
Moonrunners the warriors Tram 2 paris
Tram 2 paris Avenue de france
Avenue de france Uottawa ste
Uottawa ste Cer lanoy
Cer lanoy 2150 islington avenue
2150 islington avenue 5 avenue des tilleuls
5 avenue des tilleuls Jostens yearbook avenue.com
Jostens yearbook avenue.com Tko je sudionik u prometu na cesti
Tko je sudionik u prometu na cesti Kako se morate ponašati ako susretnete vozila pod pratnjom
Kako se morate ponašati ako susretnete vozila pod pratnjom 65 avenue de limoges niort
65 avenue de limoges niort Rose avenue church of christ
Rose avenue church of christ 160 5th ave nyc
160 5th ave nyc Ste en
Ste en Cenavenue
Cenavenue Gianotti-crosti incubation
Gianotti-crosti incubation Derek glaaser
Derek glaaser Na čo by ste použili almaru
Na čo by ste použili almaru 590 fifth avenue
590 fifth avenue Second avenue subway phase 3 timeline
Second avenue subway phase 3 timeline