2 4 Chemical Reactions Enzymes chemical reaction process




- Slides: 12

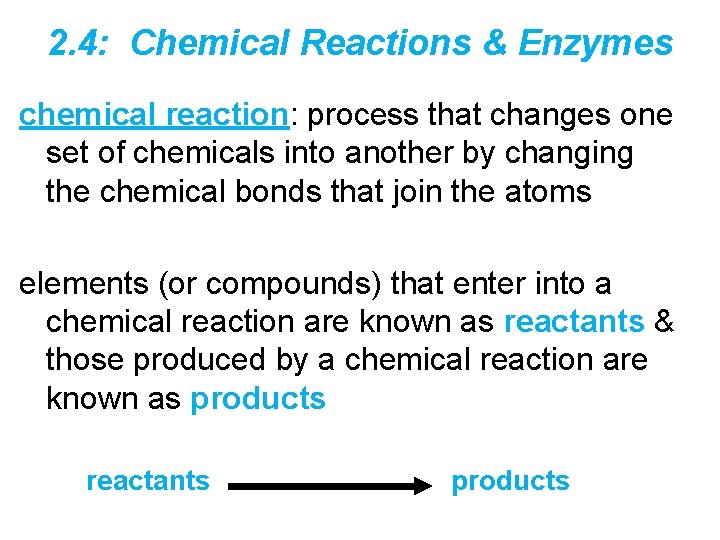
2. 4: Chemical Reactions & Enzymes chemical reaction: process that changes one set of chemicals into another by changing the chemical bonds that join the atoms elements (or compounds) that enter into a chemical reaction are known as reactants & those produced by a chemical reaction are known as products reactants products

energy is released or absorbed during chemical reactions that release energy usually occur on their own chemical reactions that absorb energy will not occur without a source of energy

every organism must have a source of energy to carry out the chemical reactions it needs to stay alive plants get their energy by trapping and storing the energy from sunlight in energy-rich compounds animals get their energy when they eat plants or other animals

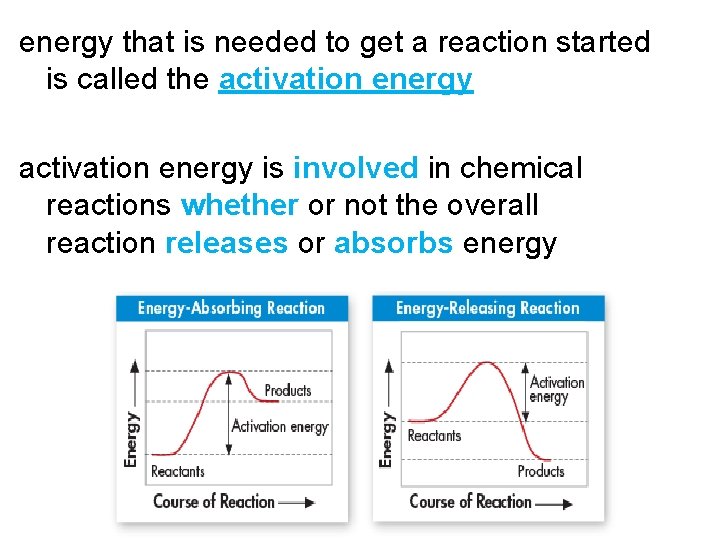
energy that is needed to get a reaction started is called the activation energy is involved in chemical reactions whether or not the overall reaction releases or absorbs energy

some chemical reactions are too slow or have activation energies that are too high to make them practical for living organisms these chemical reactions are made possible by catalysts catalyst: substance that speeds up the rate of a chemical reaction work by lowering a reaction’s activation energy

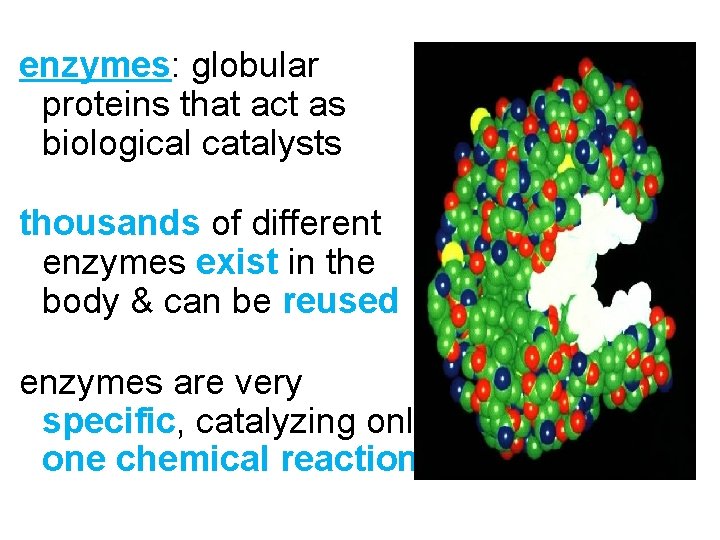
enzymes: globular proteins that act as biological catalysts thousands of different enzymes exist in the body & can be reused enzymes are very specific, catalyzing only one chemical reaction

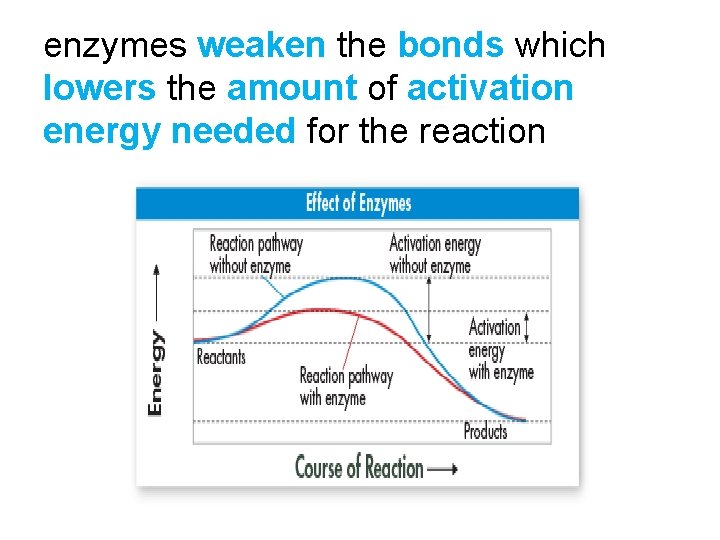
enzymes weaken the bonds which lowers the amount of activation energy needed for the reaction

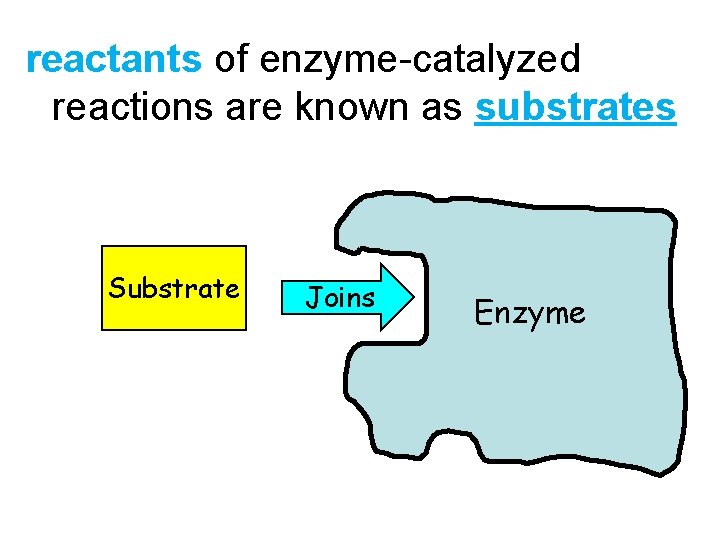
reactants of enzyme-catalyzed reactions are known as substrates Substrate Joins Enzyme 8

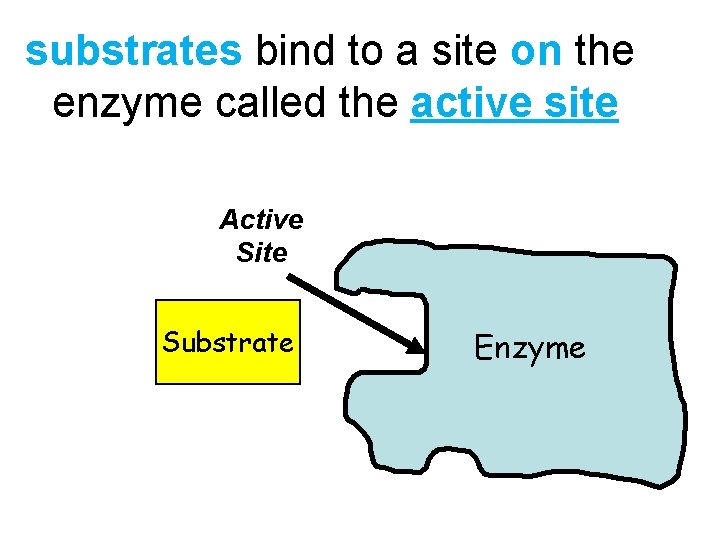
substrates bind to a site on the enzyme called the active site Active Site Substrate Enzyme 9

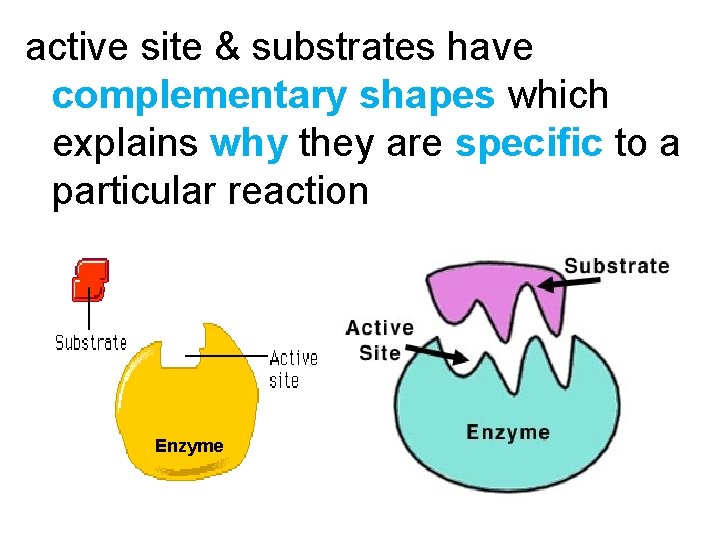
active site & substrates have complementary shapes which explains why they are specific to a particular reaction Enzyme

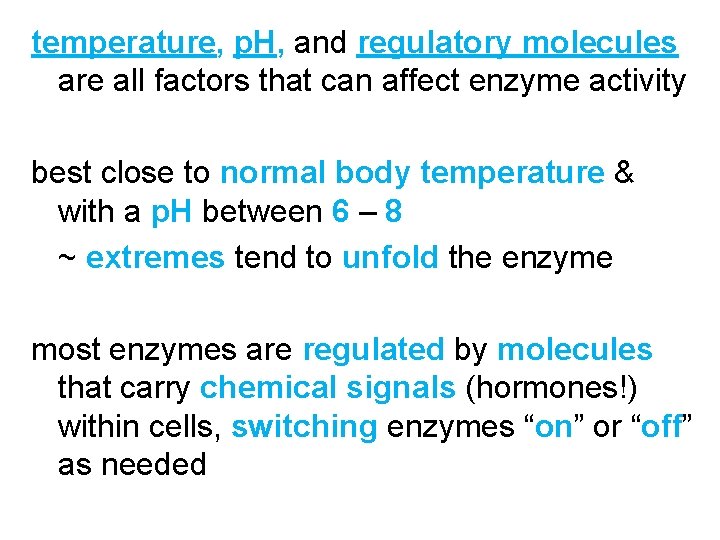
temperature, p. H, and regulatory molecules are all factors that can affect enzyme activity best close to normal body temperature & with a p. H between 6 – 8 ~ extremes tend to unfold the enzyme most enzymes are regulated by molecules that carry chemical signals (hormones!) within cells, switching enzymes “on” or “off” as needed

Enzyme Animation: http: //highered. mheducation. com /sites/0072495855/student_view 0/chapter 2/animation__how_enz ymes_work. html
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Www.biology-roots.com
Www.biology-roots.com Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Enzymes speed up chemical reactions by ____
Enzymes speed up chemical reactions by ____ Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Half life formula
Half life formula An example of redox reaction
An example of redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet