Working with CRN Eastern Pre Post Funding Ruth














- Slides: 16

Working with CRN Eastern Pre & Post Funding Ruth Hudson Research Delivery Manager Clinical Research Network Eastern

The NIHR

Your Local Clinical Research Network North East and North Cumbria North West Coast Yorkshire and Humber Greater Manchester East Midlands West of England Thames Valley and South Midlands Eastern Kent, Surrey and Sussex Wessex South West Peninsula North Thames South London North West London

Study Support Service

Study Support Service Double click on the black square below to start the video

Early Contact & Engagement What is it? Engage with us from the outset and gain access to free support What do we do in CRN Eastern? ● We are your Aco. RD specialists. We can provide advice on cost attribution and validate the So. ECAT at grant stage ● Provide advice on study feasibility in a variety of settings including NHS sites, Primary Care, Care Homes, Pharmacies ● NEW! We support research in non-NHS settings ● Advice around study set-up including local intelligence ● … and much more - Remember this is a tailored service so unlikely to look the same for every study!

Optimising Delivery The CRN supports studies by: • Funding research support posts in the NHS and beyond, providing training - ensuring researchers have access to experienced frontline staff • Helping identify and recruit patients, enabling researchers to complete their study on time and on target • Finding research sites across all sectors • National reach to support multi-site studies

NIHR’s Response to COVID-19 • Please see website https: //www. nihr. ac. uk/covid-19/ • All LCRNs are prioritisting the set-up and delivery of urgent public health research. • https: //www. recoverytrial. net/ • Latest news • • 142 Trusts in England participating 6, 633 participants have been enrolled

Is my study eligible for CRN support? Key eligibility criteria: • Your project must fit the DHSC definition of research • Funding must be awarded in national open competition e. g. NIHR, NIHR Non-Commercial Partners, Industry • Independent peer review required My study is funded as part of a Research Training Award am I eligible for CRN support? • Yes! If the criteria above are met • Contact us to find out more Email: studysupportservice. eastern@nihr. ac. uk

How to apply for CRN support Two routes: • Via IRAS - Answering question 5 b on the IRAS Project Filter • When HRA approval not required or study already submitted to IRAS - through the local CRN studysupportservice. eastern@nihr. ac. uk Documentation needed • Study Protocol • Grant Award letter(s) • Evidence of appropriate research approvals

DHSC - Policy Context Prof Chris Whitty - Chief Scientific Advisor DHSC. “research should be conducted with and within the populations most affected, . . . with the greatest health needs ”

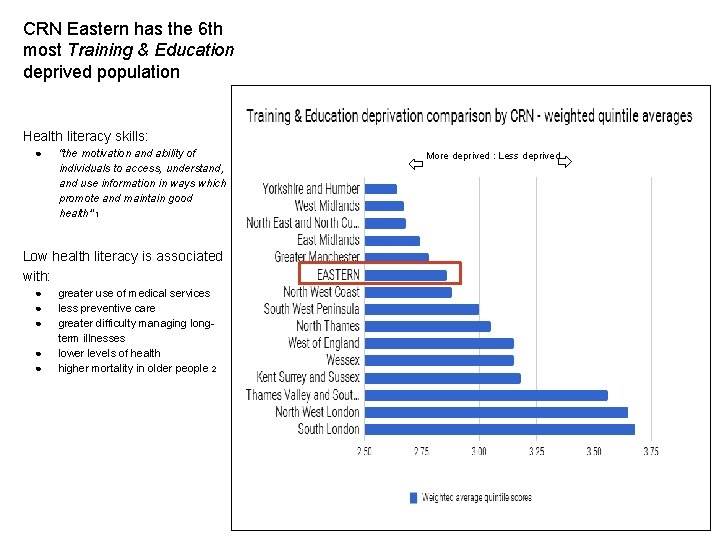
CRN Eastern has the 6 th most Training & Education deprived population Health literacy skills: ● “the motivation and ability of individuals to access, understand, and use information in ways which promote and maintain good health’” 1 Low health literacy is associated with: ● ● ● greater use of medical services less preventive care greater difficulty managing longterm illnesses lower levels of health higher mortality in older people 2 More deprived : Less deprived

Improving Access - Widening Participation • Improving the informed consent process https: //clinicaltrials. healthandcarevideos. com/ • Study promotion in local media • Social media as a recruitment tool • Empowering patients, carers & the public

Double click on the black square below to start the video

Our High Level Objectives - Your study success HLO 1 - the numbers of participants HLO 2 - recruitment to time & target HLO 6 - widening participation by enabling the involvement of range of health & social care providers HLO 7 - deliver significant levels of participation in dementia & neurodegenerative studies HLO 8 - demonstrate to people taking part in health and social care studies their contribution is valued. HLO 9 - reduce study start up times

Thank you for listening Contact us: ruth. hudson@nihr. ac. uk studysupportservice. eastern@nihr. ac. uk christian. sparke@nihr. ac. uk (CUH So. ECATs)