Why do some reactions happen and others dont





![Initial Rate Method: Example 2 NOTICE: When [O 2] is doubled without changing [NO], Initial Rate Method: Example 2 NOTICE: When [O 2] is doubled without changing [NO],](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-18.jpg)
![Initial Rate Method: Example 2 NOTICE: When [NO] is doubled without changing [O 2], Initial Rate Method: Example 2 NOTICE: When [NO] is doubled without changing [O 2],](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-19.jpg)
![Initial Rate Method: Example 2 The reaction is first order in [O 2] and Initial Rate Method: Example 2 The reaction is first order in [O 2] and](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-20.jpg)
![Example 2. A B The reaction is first order. If [A] is initially 0. Example 2. A B The reaction is first order. If [A] is initially 0.](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-23.jpg)
![Graphical Method for Determining Rate Laws How it works: 1. Collect [R] over an Graphical Method for Determining Rate Laws How it works: 1. Collect [R] over an](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-26.jpg)
![Graphical Method for Determining Rate Laws Rate = k[H 2 O 2] k = Graphical Method for Determining Rate Laws Rate = k[H 2 O 2] k =](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-31.jpg)

- Slides: 43

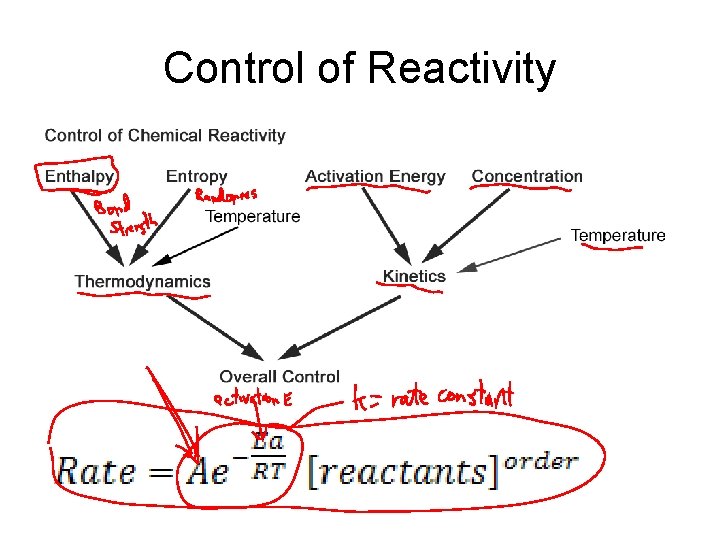
Why do some reactions happen and others don’t? Are the products more stable than the reactants? Thermodynamics Does the reaction go at a reasonable rate? Kinetics

What would affect how fast a reaction happens?

Control of Reactivity

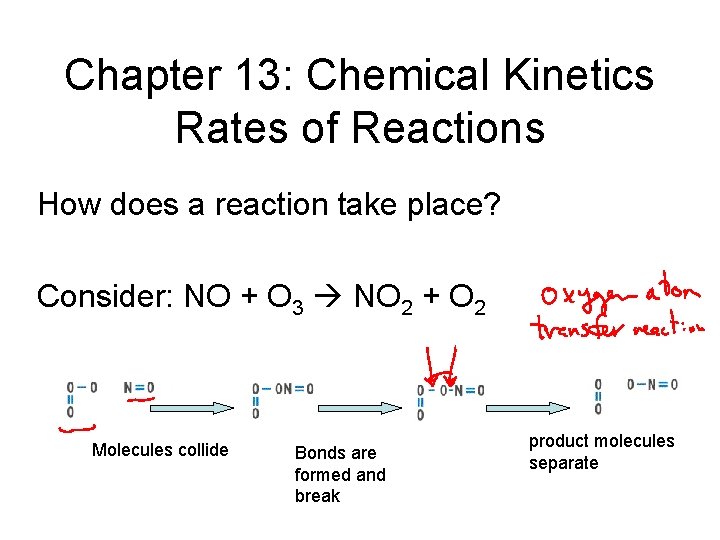
Chapter 13: Chemical Kinetics Rates of Reactions How does a reaction take place? Consider: NO + O 3 NO 2 + O 2 Molecules collide Bonds are formed and break product molecules separate

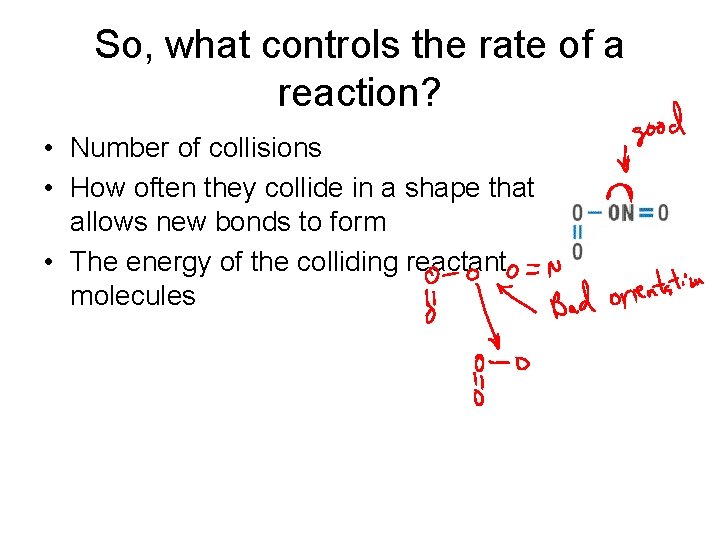
So, what controls the rate of a reaction? • Number of collisions • How often they collide in a shape that allows new bonds to form • The energy of the colliding reactant molecules

Collision Theory For a reaction to take place: • Molecules must collide • They must do so in the correct orientation • They must collide with an energy greater than the “activation” energy

Concentration Dependence • It makes sense that as concentration increases, the number of collisions per second will increase • Therefore, in general, as concentration increases, rate increases • But, it depends on which collisions control the rate • So, you can’t predict concentration dependence- it must be measured experimentally

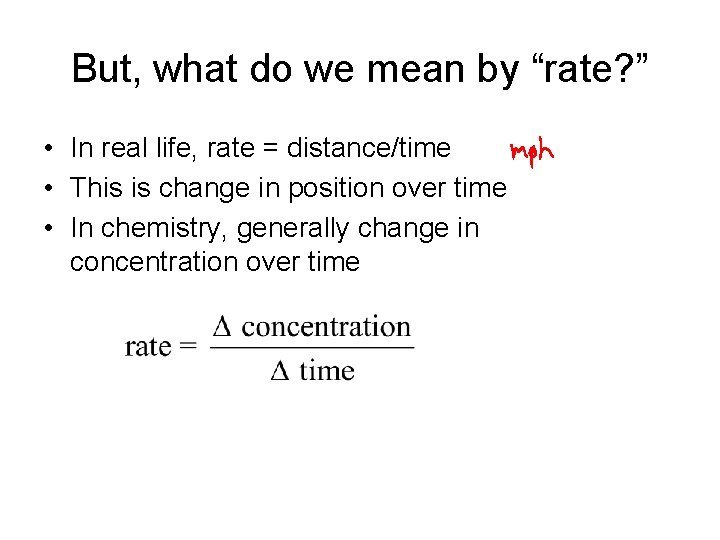
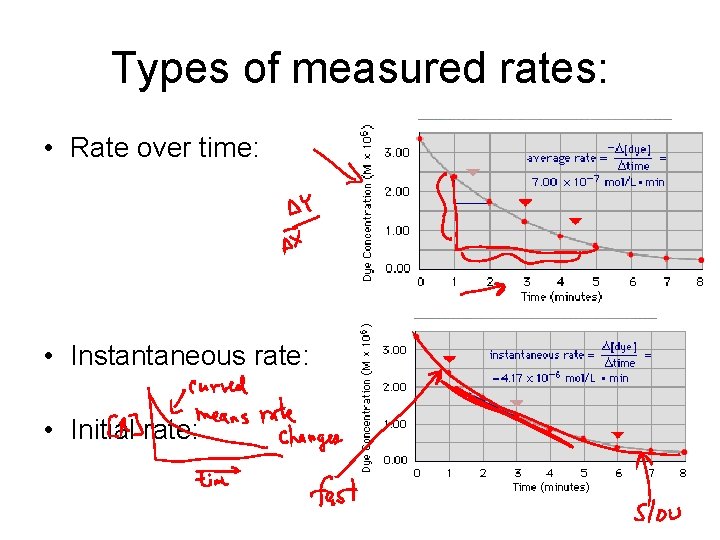
But, what do we mean by “rate? ” • In real life, rate = distance/time • This is change in position over time • In chemistry, generally change in concentration over time

Types of measured rates: • Rate over time: • Instantaneous rate: • Initial rate:

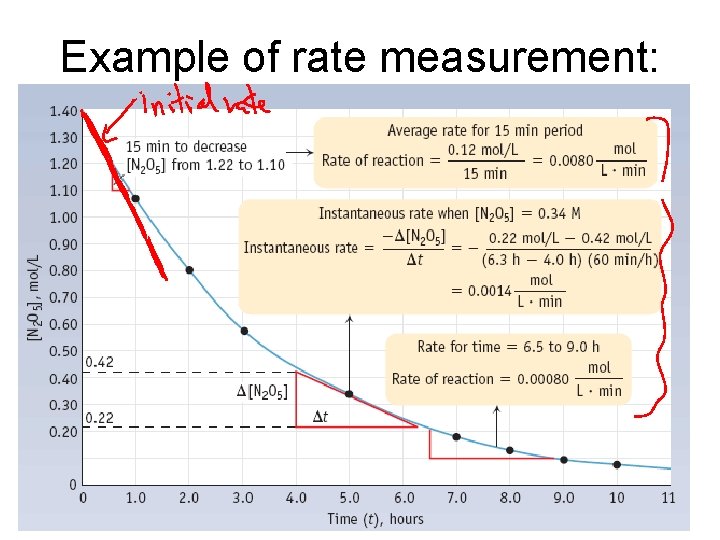
Example of rate measurement:

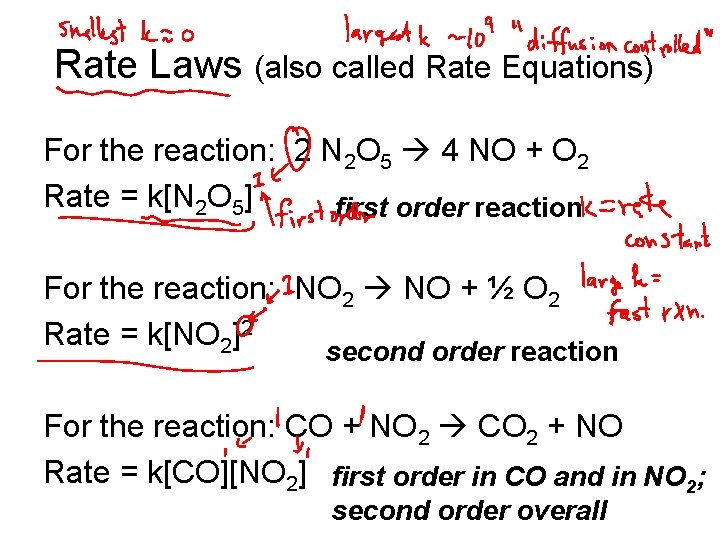
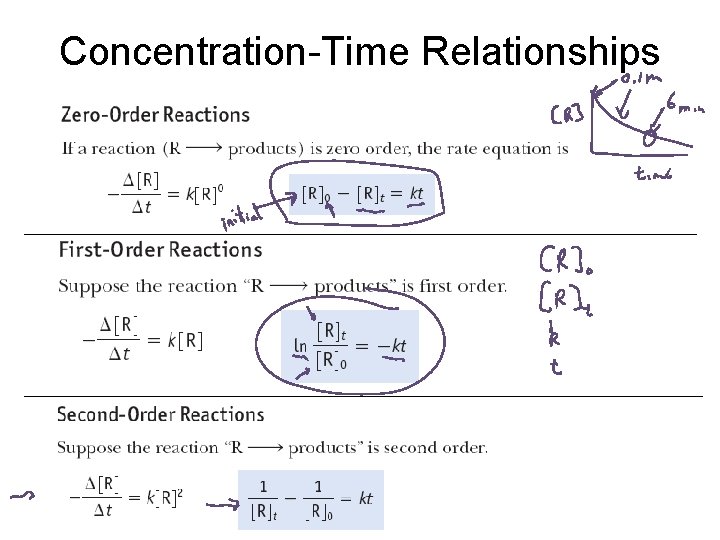
Rate Laws (also called Rate Equations) For the reaction: 2 N 2 O 5 4 NO + O 2 Rate = k[N 2 O 5] first order reaction For the reaction: NO 2 NO + ½ O 2 Rate = k[NO 2]2 second order reaction For the reaction: CO + NO 2 CO 2 + NO Rate = k[CO][NO 2] first order in CO and in NO 2; second order overall

Determining a Rate Law • Remember– it must be done by experiment; the reaction equation does not tell you the rate law • Two methods: Initial Rates Graphical Method

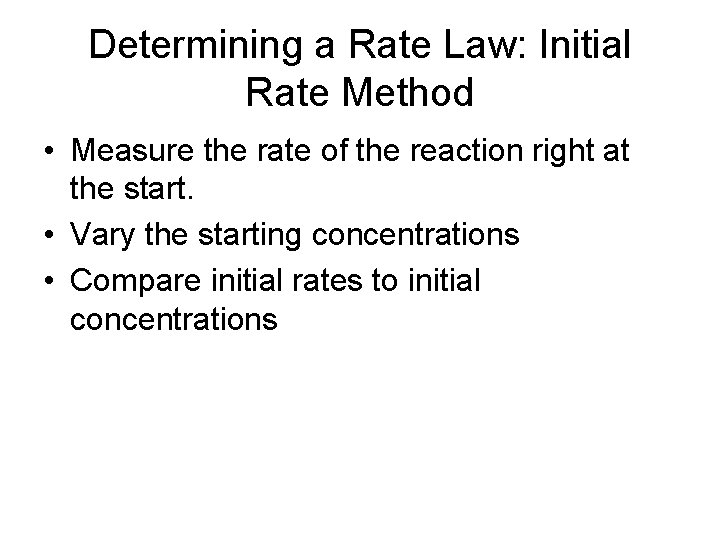
Determining a Rate Law: Initial Rate Method • Measure the rate of the reaction right at the start. • Vary the starting concentrations • Compare initial rates to initial concentrations

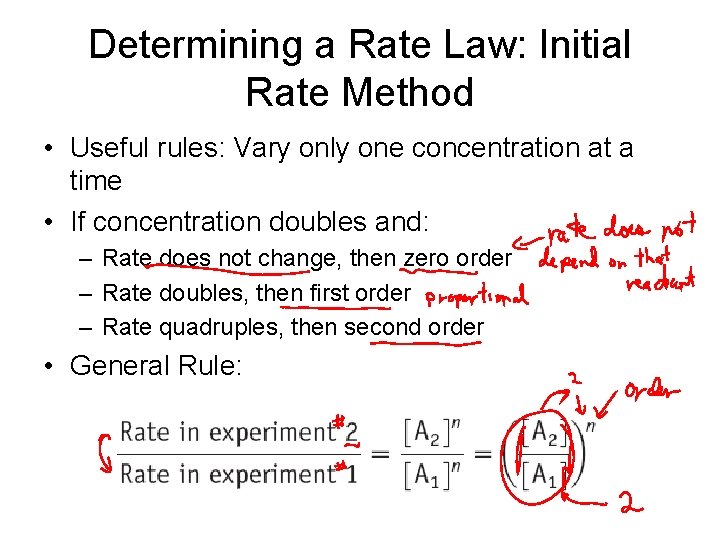
Determining a Rate Law: Initial Rate Method • Useful rules: Vary only one concentration at a time • If concentration doubles and: – Rate does not change, then zero order – Rate doubles, then first order – Rate quadruples, then second order • General Rule:

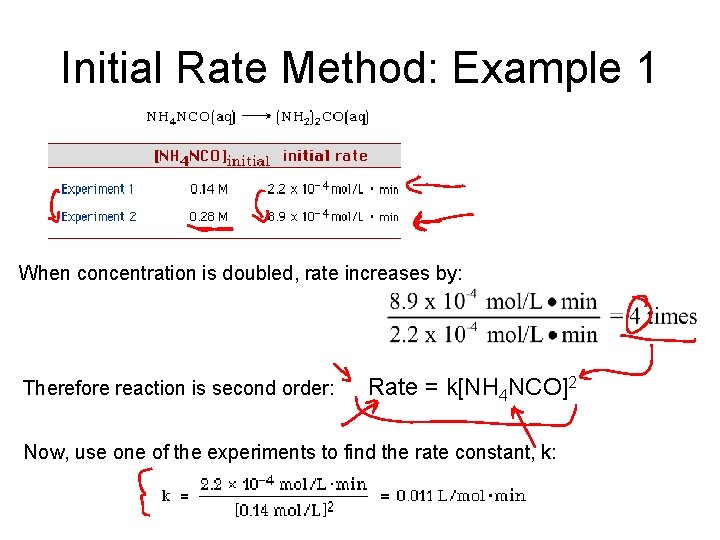
Initial Rate Method: Example 1 When concentration is doubled, rate increases by: Therefore reaction is second order: Rate = k[NH 4 NCO]2 Now, use one of the experiments to find the rate constant, k:

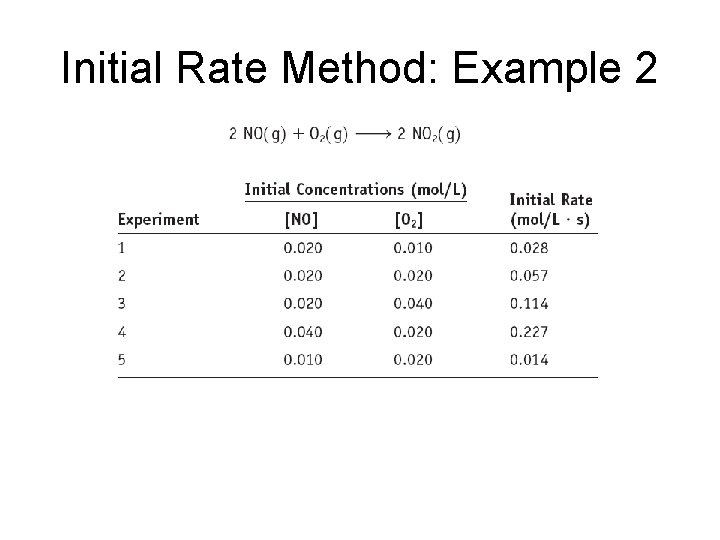
Initial Rate Method: Example 2

![Initial Rate Method Example 2 NOTICE When O 2 is doubled without changing NO Initial Rate Method: Example 2 NOTICE: When [O 2] is doubled without changing [NO],](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-18.jpg)
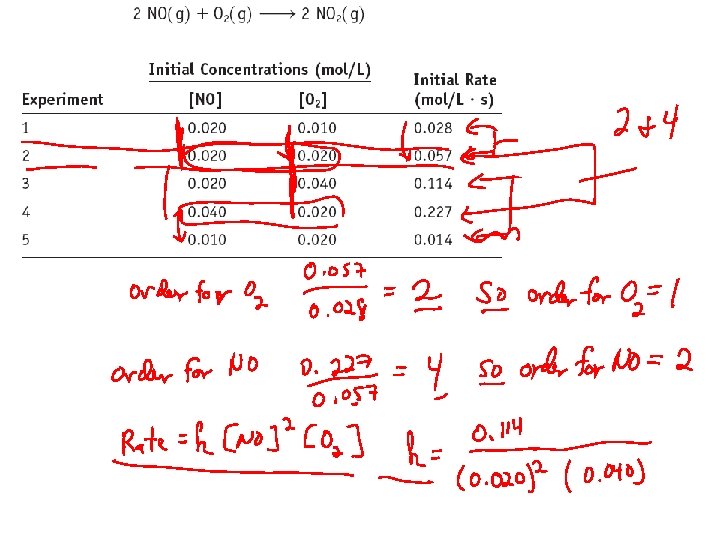
Initial Rate Method: Example 2 NOTICE: When [O 2] is doubled without changing [NO], the rate doubles. Therefore the reaction is first order in [O 2].
![Initial Rate Method Example 2 NOTICE When NO is doubled without changing O 2 Initial Rate Method: Example 2 NOTICE: When [NO] is doubled without changing [O 2],](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-19.jpg)
Initial Rate Method: Example 2 NOTICE: When [NO] is doubled without changing [O 2], the rate quadruples. Therefore the reaction is second order in [NO].
![Initial Rate Method Example 2 The reaction is first order in O 2 and Initial Rate Method: Example 2 The reaction is first order in [O 2] and](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-20.jpg)
Initial Rate Method: Example 2 The reaction is first order in [O 2] and second order in [NO]. Rate = k[O 2][NO]2 Now we find the value of k.

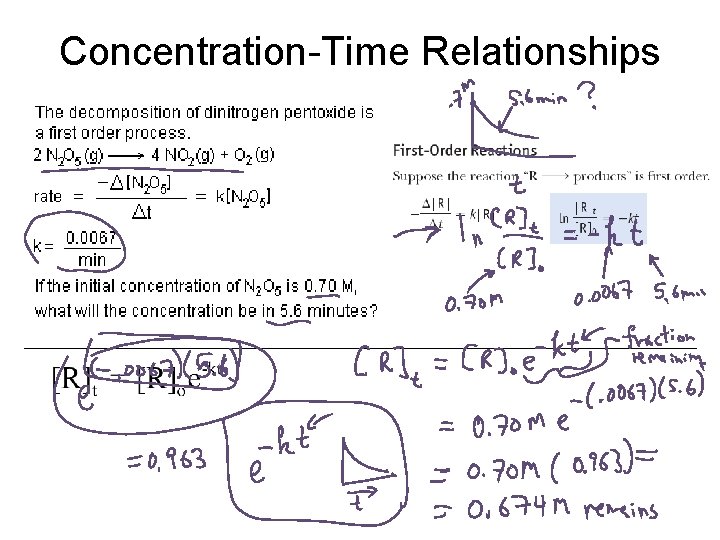
Concentration-Time Relationships

Concentration-Time Relationships
![Example 2 A B The reaction is first order If A is initially 0 Example 2. A B The reaction is first order. If [A] is initially 0.](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-23.jpg)
Example 2. A B The reaction is first order. If [A] is initially 0. 100 M and after 18 minutes, [A] has dropped to 0. 064 M, what is k?

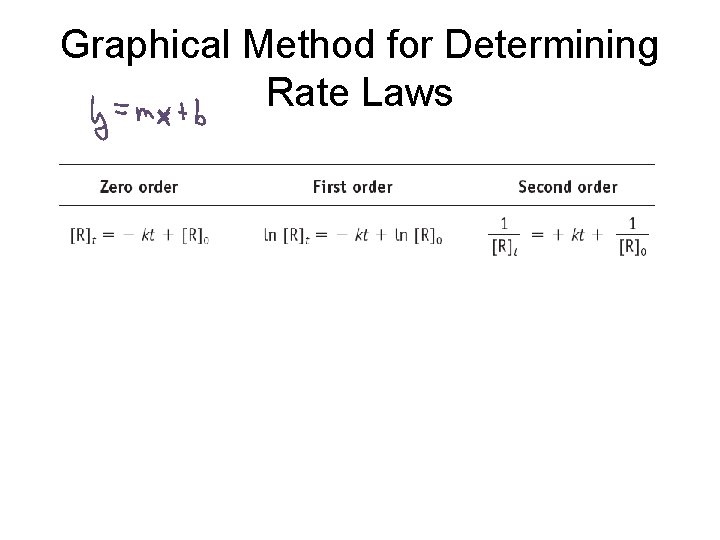
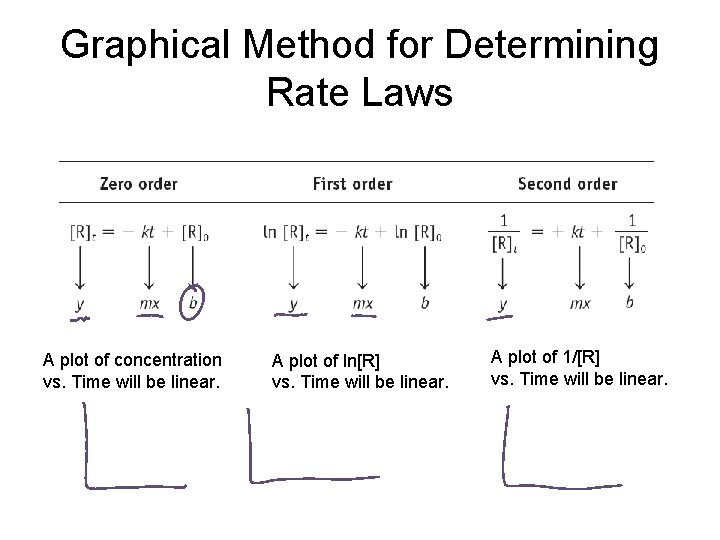
Graphical Method for Determining Rate Laws

Graphical Method for Determining Rate Laws A plot of concentration vs. Time will be linear. A plot of ln[R] vs. Time will be linear. A plot of 1/[R] vs. Time will be linear.
![Graphical Method for Determining Rate Laws How it works 1 Collect R over an Graphical Method for Determining Rate Laws How it works: 1. Collect [R] over an](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-26.jpg)
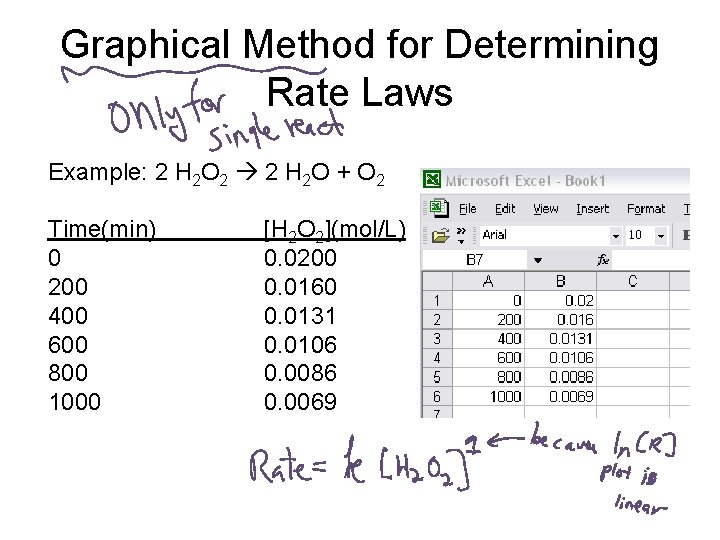
Graphical Method for Determining Rate Laws How it works: 1. Collect [R] over an interval of times. 2. Make plots of [R] vs. time ln[R] vs. time 1/R vs. time Only one will be linear. That tells you the reaction order. The slope of the linear plot is the rate constant.

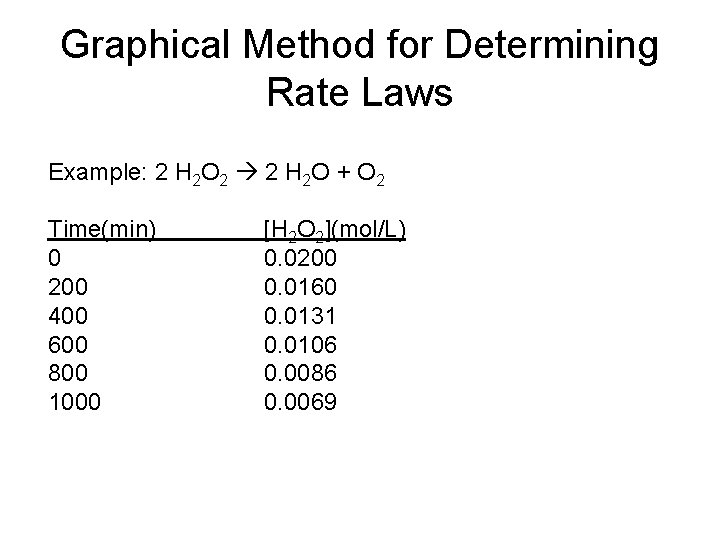
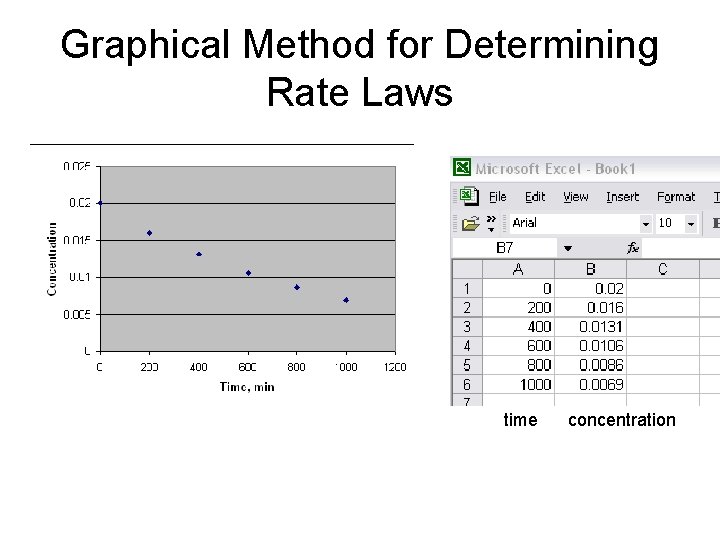
Graphical Method for Determining Rate Laws Example: 2 H 2 O 2 2 H 2 O + O 2 Time(min) 0 200 400 600 800 1000 [H 2 O 2](mol/L) 0. 0200 0. 0160 0. 0131 0. 0106 0. 0086 0. 0069

Graphical Method for Determining Rate Laws Example: 2 H 2 O 2 2 H 2 O + O 2 Time(min) 0 200 400 600 800 1000 [H 2 O 2](mol/L) 0. 0200 0. 0160 0. 0131 0. 0106 0. 0086 0. 0069

Graphical Method for Determining Rate Laws time concentration

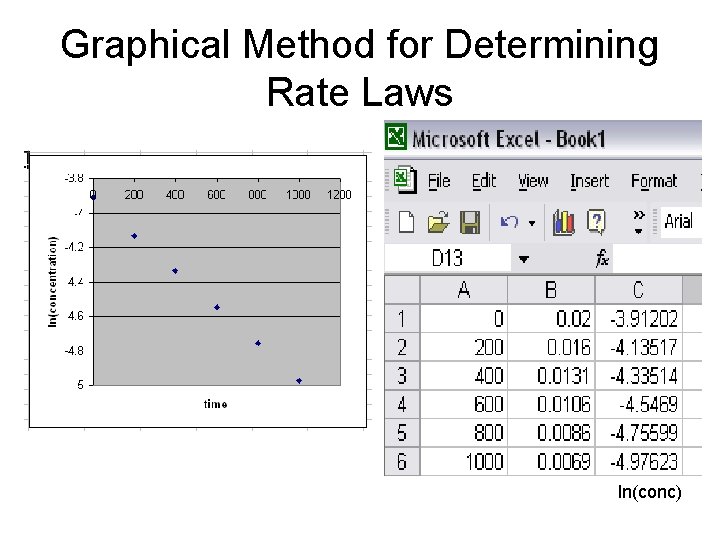
Graphical Method for Determining Rate Laws ln(conc)
![Graphical Method for Determining Rate Laws Rate kH 2 O 2 k Graphical Method for Determining Rate Laws Rate = k[H 2 O 2] k =](https://slidetodoc.com/presentation_image_h/0ca0d4825865a97b3c31940617431513/image-31.jpg)
Graphical Method for Determining Rate Laws Rate = k[H 2 O 2] k = 0. 0011 min-1 ln(conc)

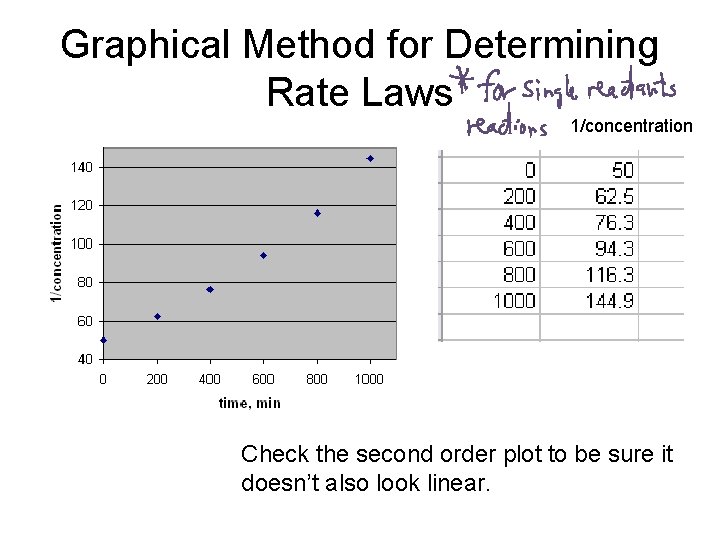
Graphical Method for Determining Rate Laws 1/concentration Check the second order plot to be sure it doesn’t also look linear.

Half-Life • Half-Life = the time it takes for half the reactant concentration to drop to half of its original value

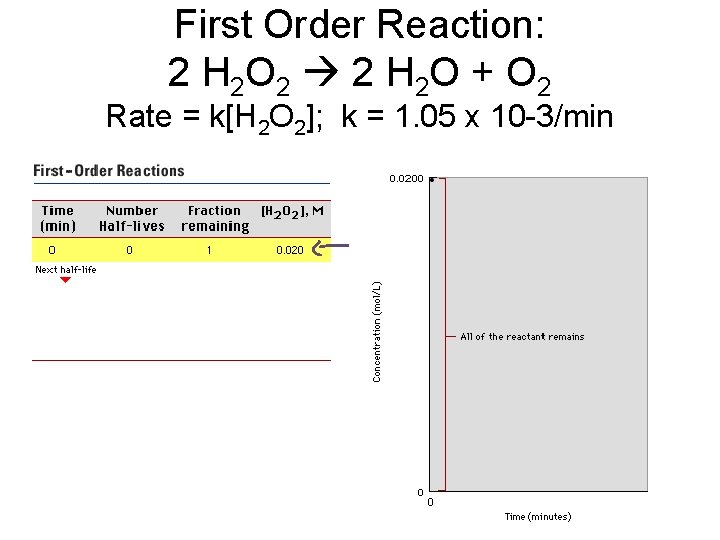
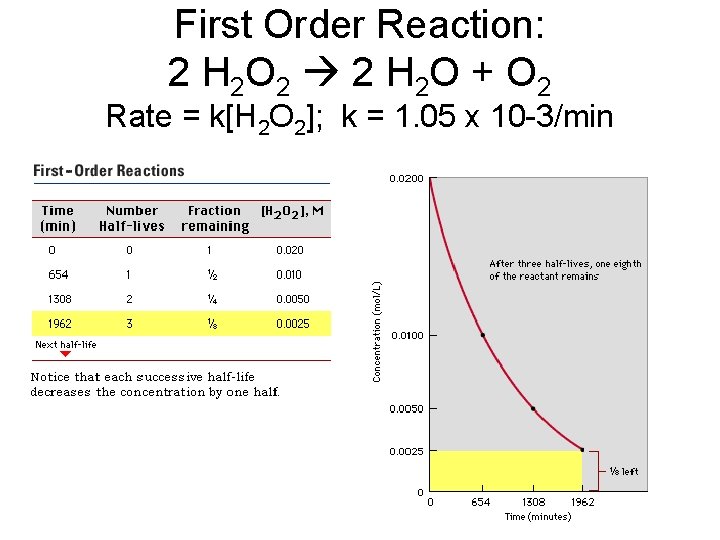
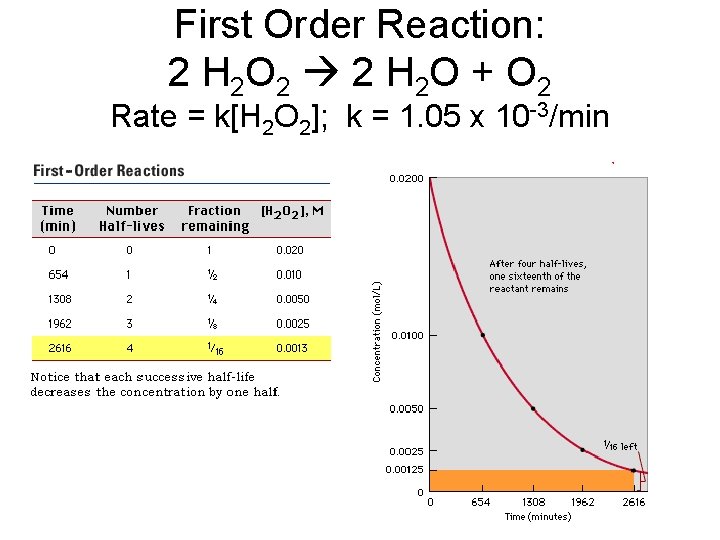
First Order Reaction: 2 H 2 O 2 2 H 2 O + O 2 Rate = k[H 2 O 2]; k = 1. 05 x 10 -3/min

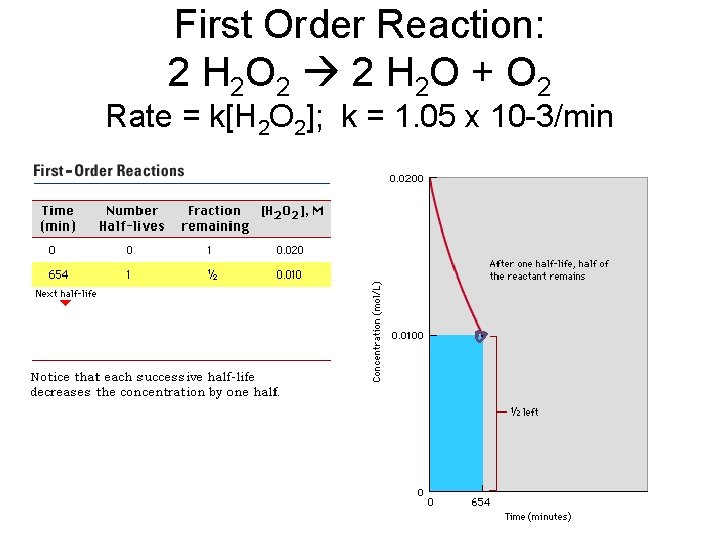
First Order Reaction: 2 H 2 O 2 2 H 2 O + O 2 Rate = k[H 2 O 2]; k = 1. 05 x 10 -3/min

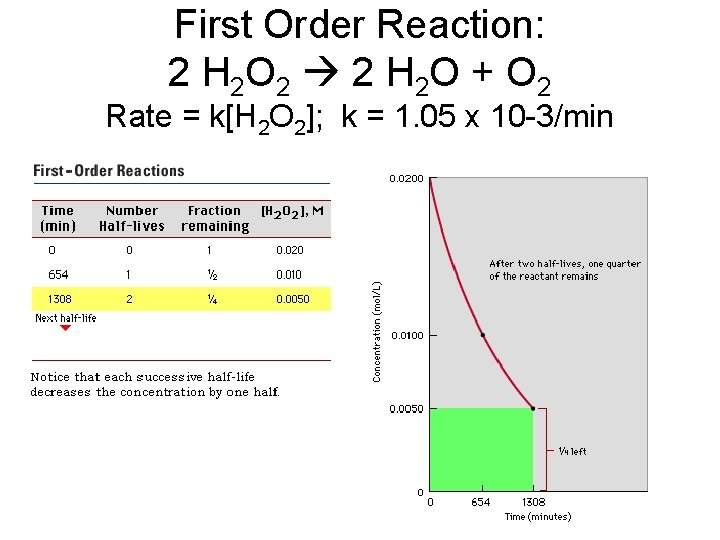
First Order Reaction: 2 H 2 O 2 2 H 2 O + O 2 Rate = k[H 2 O 2]; k = 1. 05 x 10 -3/min

First Order Reaction: 2 H 2 O 2 2 H 2 O + O 2 Rate = k[H 2 O 2]; k = 1. 05 x 10 -3/min

First Order Reaction: 2 H 2 O 2 2 H 2 O + O 2 Rate = k[H 2 O 2]; k = 1. 05 x 10 -3/min

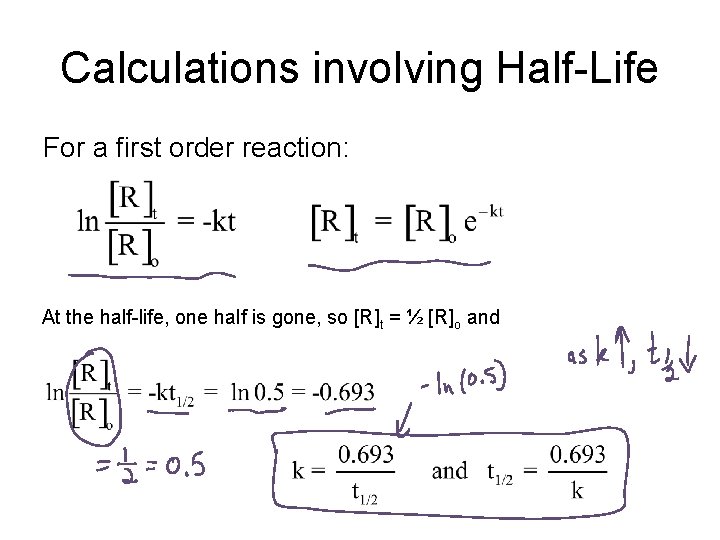
Calculations involving Half-Life For a first order reaction: At the half-life, one half is gone, so [R]t = ½ [R]o and

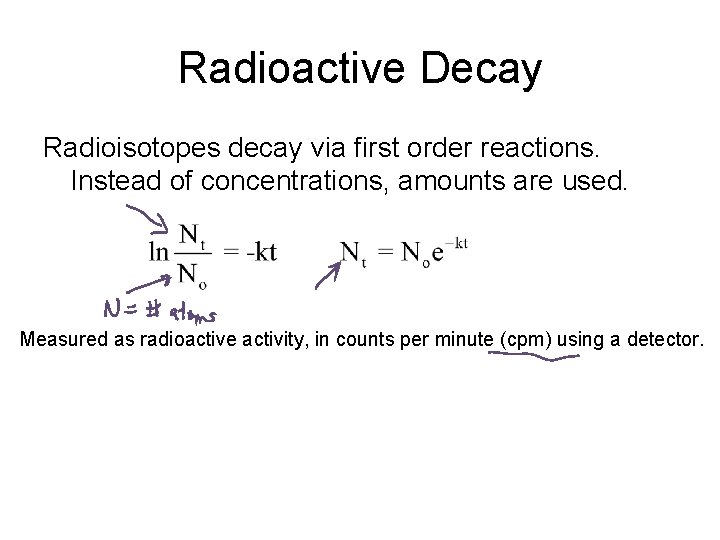
Radioactive Decay Radioisotopes decay via first order reactions. Instead of concentrations, amounts are used. Measured as radioactive activity, in counts per minute (cpm) using a detector.

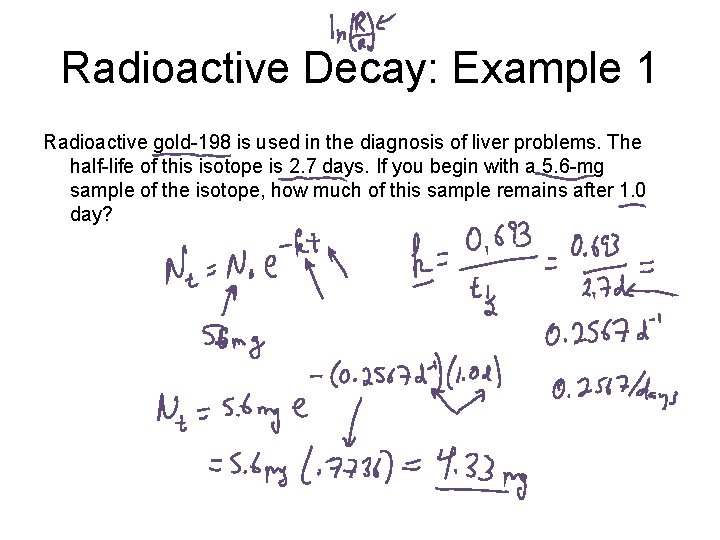
Radioactive Decay: Example 1 Radioactive gold-198 is used in the diagnosis of liver problems. The half-life of this isotope is 2. 7 days. If you begin with a 5. 6 -mg sample of the isotope, how much of this sample remains after 1. 0 day?

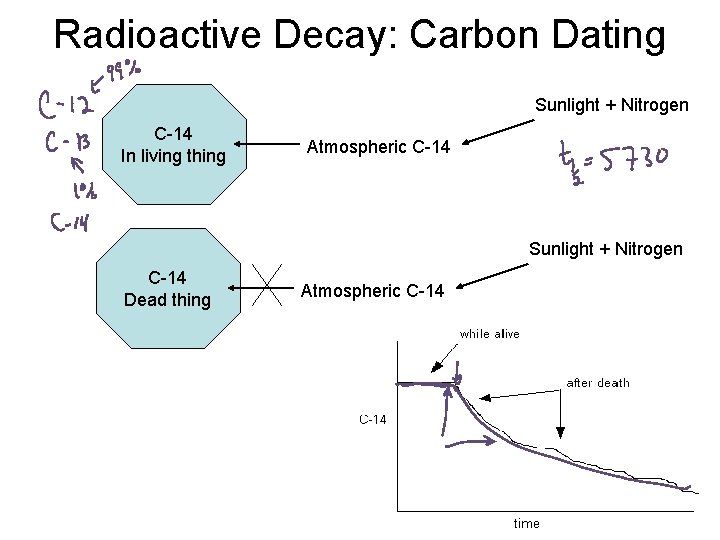
Radioactive Decay: Carbon Dating Sunlight + Nitrogen C-14 In living thing Atmospheric C-14 Sunlight + Nitrogen C-14 Dead thing Atmospheric C-14

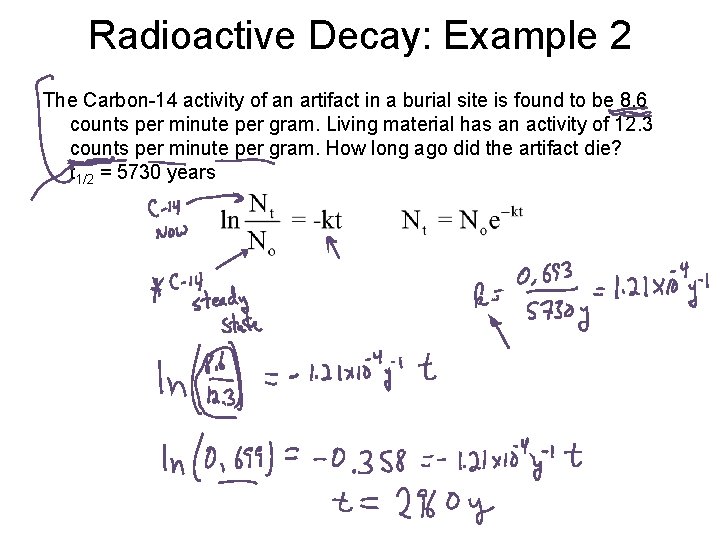
Radioactive Decay: Example 2 The Carbon-14 activity of an artifact in a burial site is found to be 8. 6 counts per minute per gram. Living material has an activity of 12. 3 counts per minute per gram. How long ago did the artifact die? t 1/2 = 5730 years