Aim 1 Why dont water and oil mix









- Slides: 13


Aim 1: Why don’t water and oil mix?


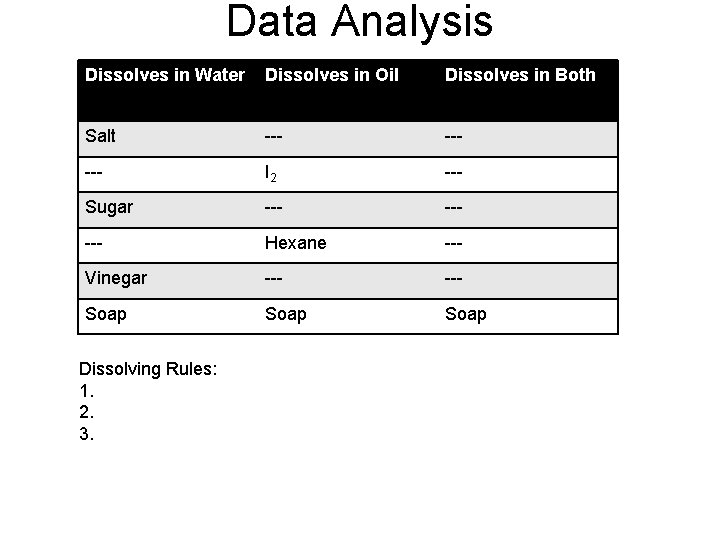
“Like Dissolves Like” Data: Na. Cl will dissolve in water, but not oil. I 2 will dissolve in oil, but not water. Sugar will dissolve in water, but not oil. Hexane will mix with oil but not water. Soap will mix with water and oil.

Data Analysis Dissolves in Water Dissolves in Oil Dissolves in Both Salt Will dissolve in --- Water --Will dissolve in I 2 Oil --- Sugar --- --- Hexane --- Vinegar --- Soap Dissolving Rules: 1. 2. 3. ---

Exit Ticket What kind of substance would be needed to clean up an oil spill in the ocean? Or If our bodies are mostly water, how do we mix with the oils we eat?

Aim 2: Why do we put salt on the roads in winter? Lesson Objectives: 1) Students should understand the effect of solutes on the boiling and freezing points of water. 2) Students should understand the relationship between the number of ions and the change in bp and fp. 3) Students should be able to determine the relative effect on bp and fp by determining the number of moles of particles produced when various compounds dissolve in water.

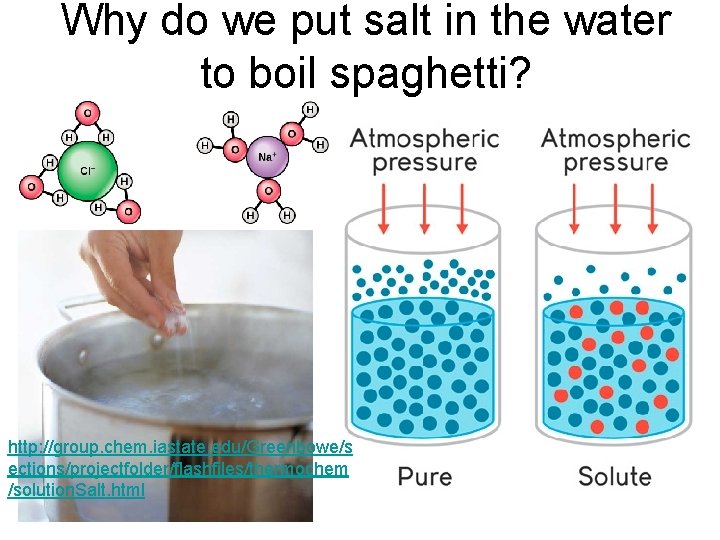
Why do we put salt in the water to boil spaghetti? http: //group. chem. iastate. edu/Greenbowe/s ections/projectfolder/flashfiles/thermochem /solution. Salt. html

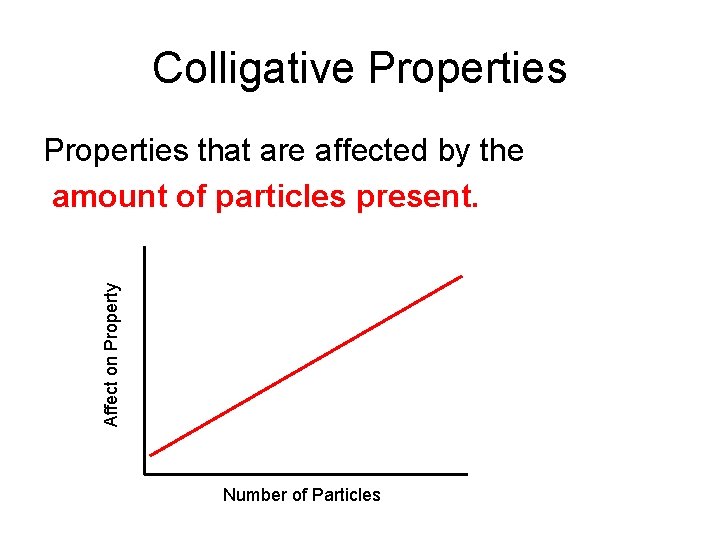
Colligative Properties Affect on Property Properties that are affected by the amount of particles present. Number of Particles

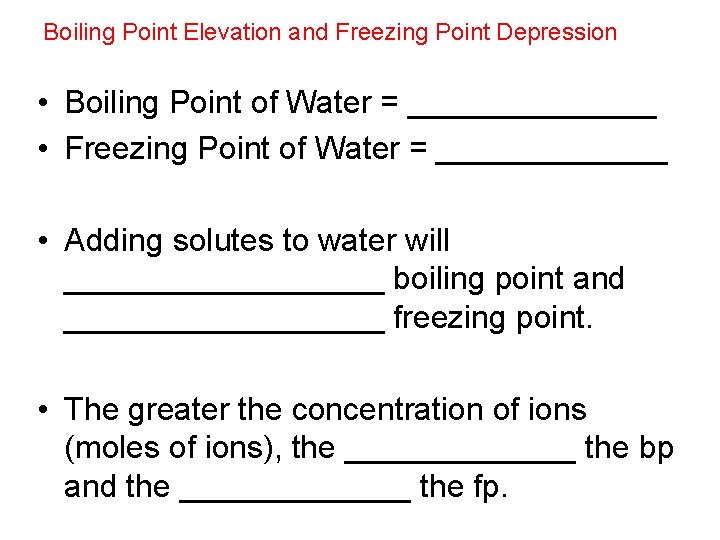
Boiling Point Elevation and Freezing Point Depression • Boiling Point of Water = _______ • Freezing Point of Water = _______ • Adding solutes to water will _________ boiling point and _________ freezing point. • The greater the concentration of ions (moles of ions), the _______ the bp and the _______ the fp.

Which of these salts would you purchase to melt the ice on your sidewalks? Ca. Cl 2 50 lb bag $20. 00 Na. Cl 50 lb bag $20. 00

Place the following in order of greatest change to bp/fp of water. Na. Cl(s) Mg. Cl 2(s) C 6 H 12 O 6(s) NH 4 NO 3(s) Ca 3(PO 4)2(s) H 3 PO 4(l)

Exit Ticket Why would we add salt to ice used in making ice cream?
 Why don't water and oil mix
Why don't water and oil mix Don't ask why why why
Don't ask why why why Dont ask dont tell political cartoon
Dont ask dont tell political cartoon Dont laugh at me dont call me names
Dont laugh at me dont call me names Water and water and water water
Water and water and water water Andreas carlsson bye bye bye
Andreas carlsson bye bye bye Oral emulsion example
Oral emulsion example Depth of product line
Depth of product line How does the cockle avoid the moon snail?
How does the cockle avoid the moon snail? A separating funnel is used to separate
A separating funnel is used to separate Oil and water analogy
Oil and water analogy Ethics is a personal and individual affair
Ethics is a personal and individual affair Mortar water ratio
Mortar water ratio Contoh produk emulsi w/o
Contoh produk emulsi w/o